Nh3 Drawing, Web nh3 (ammonia) lewis structure has a nitrogen atom (n) at the center which is surrounded by three hydrogen atoms (h).
Nh3 Drawing - This will help you understand the molecule’s electronic structure and bonding. Web in this video i draw the dot and cross diagram for nh3 (ammonia). Web learn about the dot diagram for nh3, including its lewis structure, electron arrangement, and molecular geometry. Lewis structure of nh3 can be drawn by using valence electrons of nitrogen and hydrogen atoms. Web nh3 molecular geometry. In the nh 3 lewis structure (and all structures), hydrogen goes on the outside. All the hydrogen atoms are arranged symmetrically around the nitrogen atom which forms the base, and the two nonbonding electrons form the tip which makes the molecular geometry of nh3 trigonal pyramidal. There is 1 lone pair on the nitrogen atom (n). Household ammonia is nh 3 in water or ammonium hydroxide. 159k views 4 years ago. Ammonia is a colorless gas with a distinct odor. Web learn about the dot diagram for nh3, including its lewis structure, electron arrangement, and molecular geometry. 159k views 4 years ago. These are arranged in a tetrahedral shape. Web ammonia is a colorless gas with a chemical formula nh 3. There are 3 single bonds between the nitrogen atom (n) and each hydrogen atom (h). Remember to consider the octet rule, evaluate formal charges, and adjust the structure to minimize formal charges. Here, the given molecule is nh3 (ammonia). Calculate the total number of valence electrons. This inorganic compound has a pungent smell. Remember to consider the octet rule, evaluate formal charges, and adjust the structure to minimize formal charges. There are 8 valence electrons available for the lewis structure for nh 3. Understand how the dots represent the valence electrons and how they interact to form chemical bonds. In its aqueous form, it is called ammonium hydroxide. It's not particularly difficult but. All the hydrogen atoms are arranged symmetrically around the nitrogen atom which forms the base, and the two nonbonding electrons form the tip which makes the molecular geometry of nh3 trigonal pyramidal. For resonance structures there must be a double or triple bond present, which is not the case with nh3. I also go over hybridization and bond angle. In. Web to draw the nh3 lewis structure (ammonia) involves a few straightforward steps. Web ammonia is a colorless gas with a chemical formula nh 3. It is a colorless gas with a pungent smell and is lighter than air. There are 8 valence electrons available for the lewis structure for nh 3. There are 3 single bonds between the nitrogen. It consists of hydrogen and nitrogen. In order to draw the lewis structure of nh3, first of all you have to find the total number of valence electrons present in the nh3 molecule. The nh 3 chemical name is ammonia. There is 1 lone pair on the nitrogen atom (n). Web nh3 molecular geometry. Nitrogen (n) is in group 15 of the periodic table, which means it has 5 valence electrons. The nh 3 chemical name is ammonia. Web to draw the nh3 lewis structure (ammonia) involves a few straightforward steps. Web here in this article, we discuss only the nh3 lewis dot structure, its hybridization, shape, and molecular fact in detail, and the. For resonance structures there must be a double or triple bond present, which is not the case with nh3. Ammonia has a tetrahedral molecular geometry. In the nh 3 lewis structure (and all structures), hydrogen goes on the outside. Web ammonia, also known as nh3, is a binary hydride composed of nitrogen and hydrogen atoms. Remember to consider the octet. This inorganic compound has a pungent smell. In the nh3 lewis dot structure, n formed three sigma bonds with three h atoms. There is 1 lone pair on the nitrogen atom (n). Ammonia is a colorless gas with a distinct odor. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. Web ammonia is a colorless gas with a chemical formula nh 3. Household ammonia is nh 3 in water or ammonium hydroxide. There are 3 single bonds between the nitrogen atom (n) and each hydrogen atom (h). Web to draw the nh3 lewis structure (ammonia) involves a few straightforward steps. It also is a good example of a molecule with. Web drawing the lewis structure for nh 3. In its aqueous form, it is called ammonium hydroxide. Web nh3 molecular geometry. 159k views 4 years ago. The exception, of course, being the hydrogen's. Web this nh3 lewis structure depicts the central nitrogen atom bonded to three hydrogen atoms. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. In its concentrated form, it is dangerous and caustic. Web a video explanation of how to draw the lewis dot structure for ammonia, along with information about the compound including formal charges, polarity, hybrid orbitals, shape, and bond angles. Web nh3 (ammonia) lewis structure has a nitrogen atom (n) at the center which is surrounded by three hydrogen atoms (h). Steps of drawing the lewis structure of nh3 is explained in detail in this tutorial. Join us as we delve into the intricacies of ammonia's molecular structure and learn how to draw its lewis structure. This will help you understand the molecule’s electronic structure and bonding. Nh 3 (ammonia) is a commonly tested lewis structure. Drawing the lewis structure for nh3. It consists of hydrogen and nitrogen.
Nh3 Estrutura De Lewis

Molecular Model Of Ammonia Nh3 Molecule Vector Illust vrogue.co

Draw The Lewis Structure Of Ammonia Nh3 Drawing.rjuuc.edu.np

Ammonia (NH3) molecule. Stylized skeletal formula (chemical structure

NH3 Molecular Geometry Science Education and Tutorials

The Nh3 Lewis Dot Structure Understanding The Basics vrogue.co
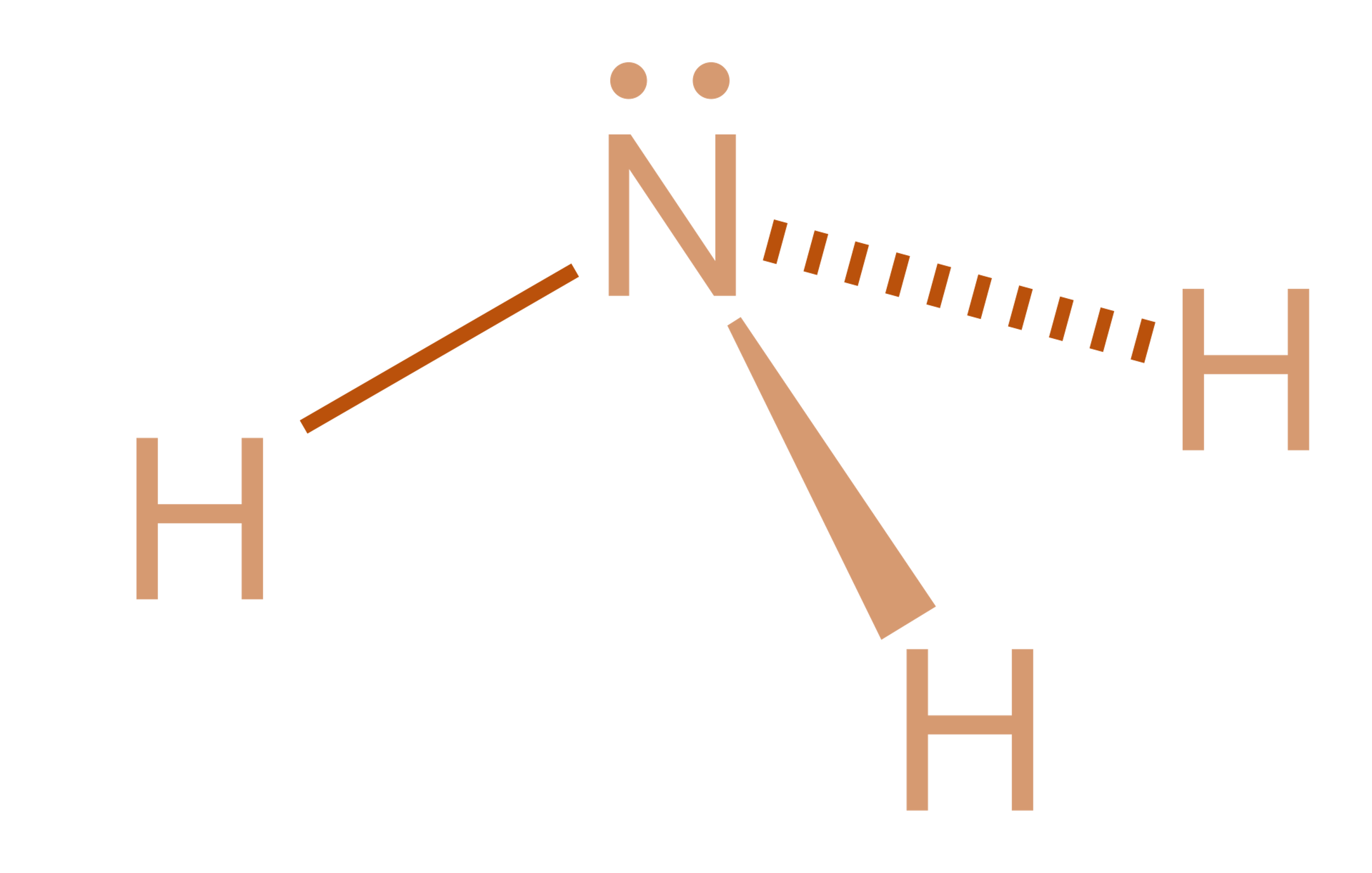
Nh3 Lewis Structure Molecular Geometry
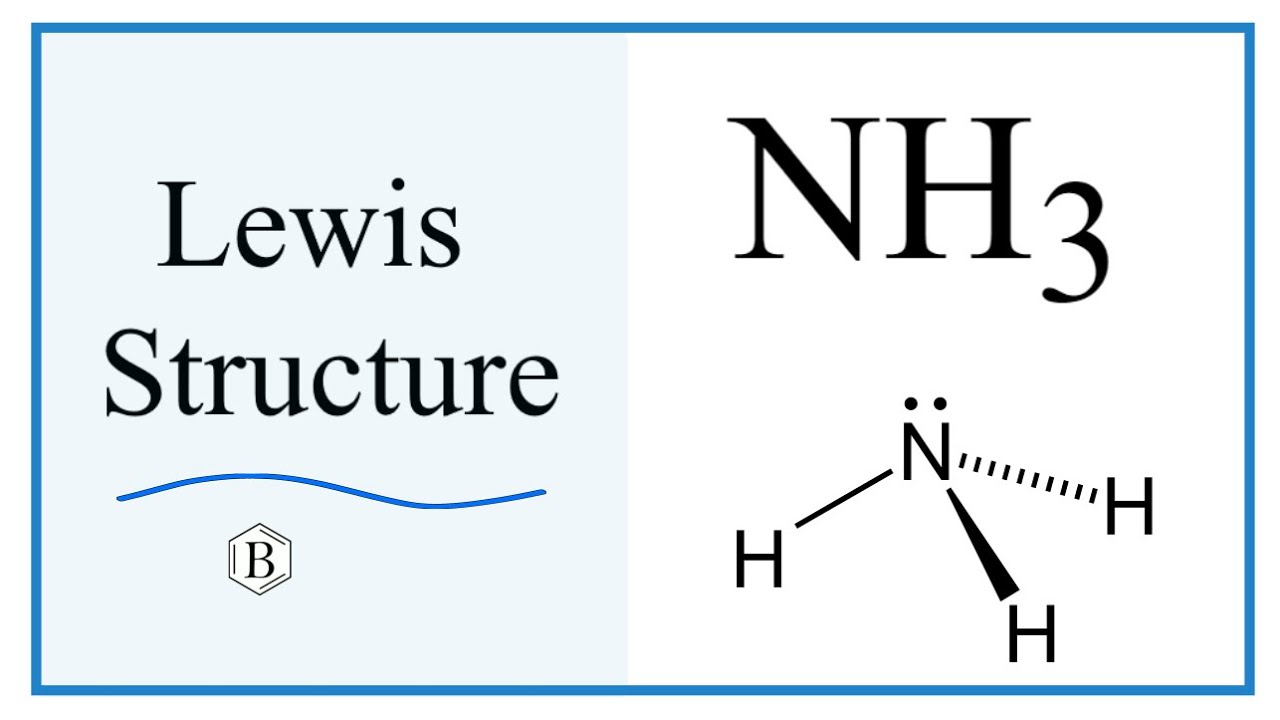
Nh3 Molecule Structure

Electron Geometry for NH3 (Ammonia) YouTube
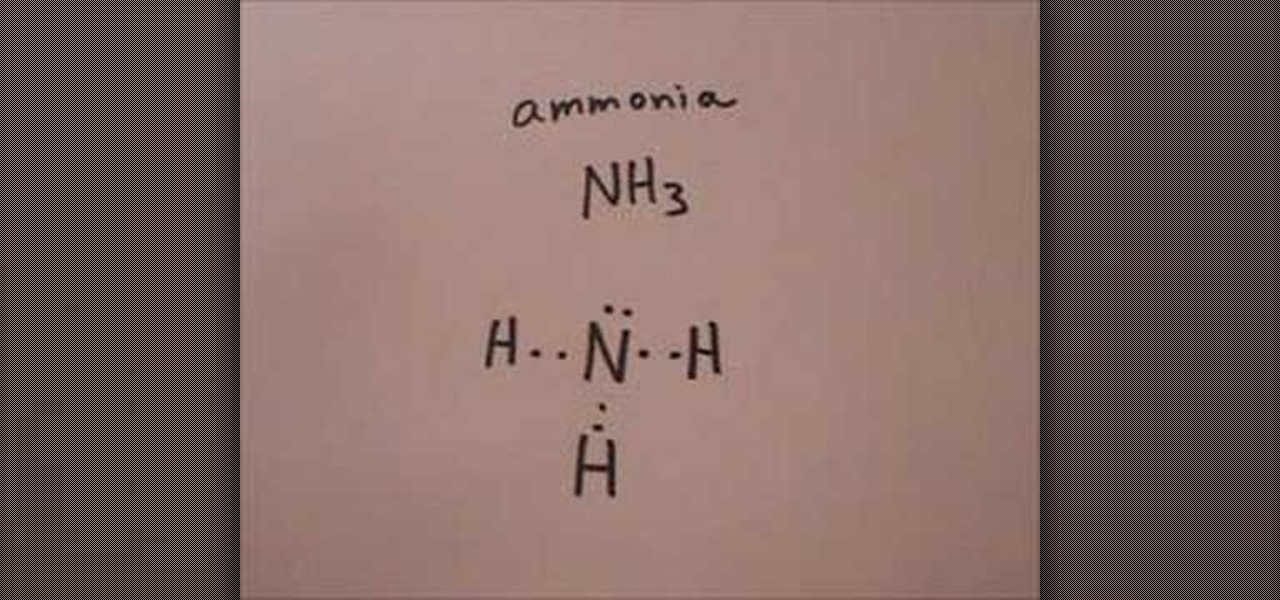
How to Draw the Lewis structure for ammonia « Science Experiments
This Inorganic Compound Has A Pungent Smell.
The Nh 3 Chemical Name Is Ammonia.
Here, The Given Molecule Is Nh3 (Ammonia).
I Also Go Over Hybridization And Bond Angle.
Related Post: