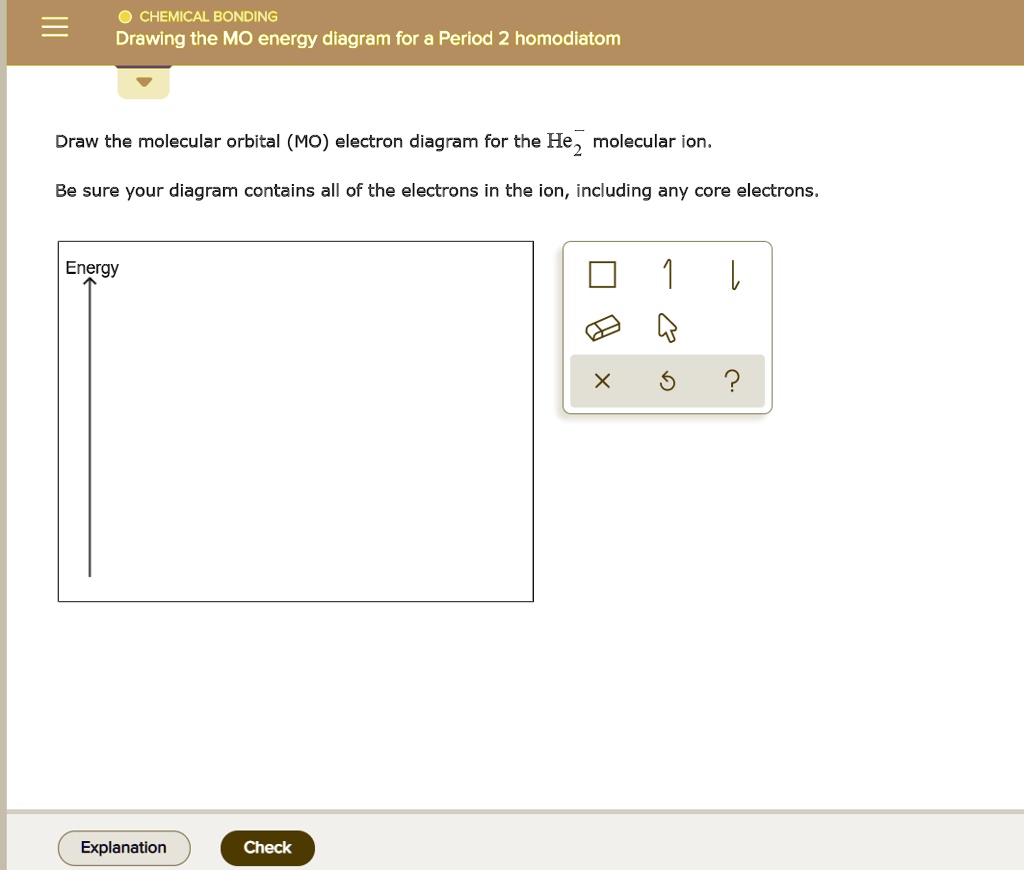
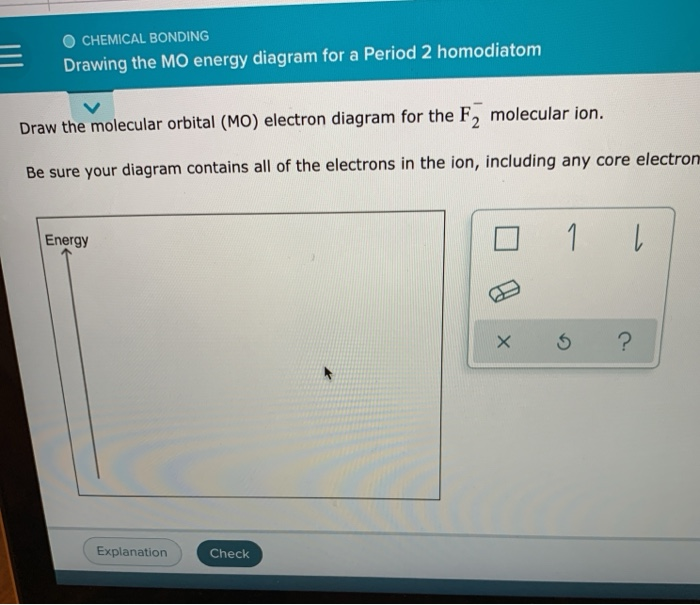
Drawing The Mo Energy Diagram For A Period 2 Homodiatom, Be sure your diagram contains all of the electrons in the ion, including any core electrons.
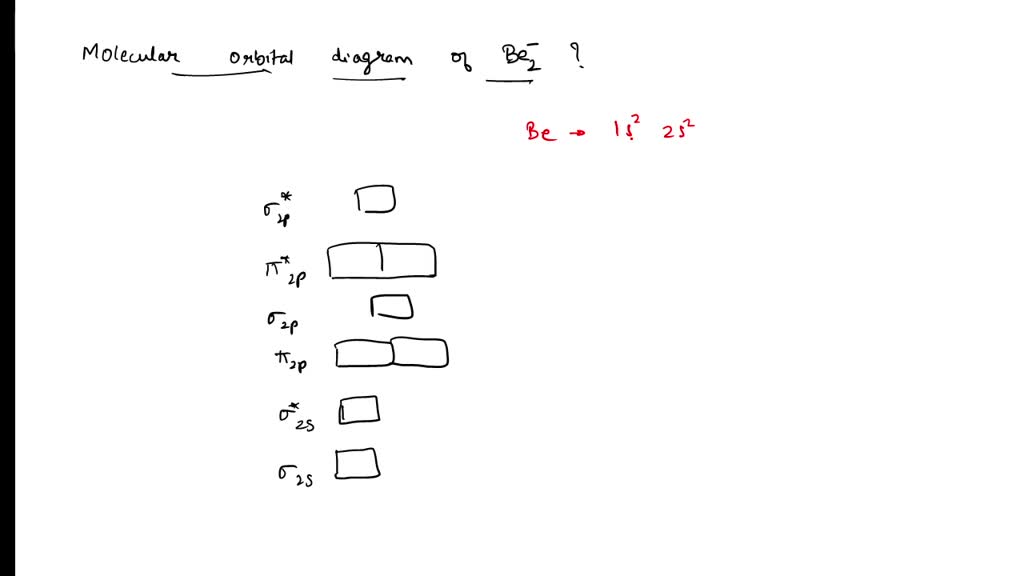
Drawing The Mo Energy Diagram For A Period 2 Homodiatom - 2 will be followed by sigma 2 and then sigma 2 pitso. Add the energies together to get the total energy for the molecule. The relative energies of the sigma orbitals drop below that of the pi orbitals'. We need to draw a diagram of the berylium 2 molecule having a negative charge. Finally, we need to draw the mo electron diagram. Then you fill those lines (molecular orbitals) with 4 electrons (two from each beryllium) there’s you’re mo diagram. Web if you have two atomic orbitals, you need two molecular orbitals. In o 2 and f 2, there is a crossover of the sigma and the pi ortbials: Draw the mo energy diagram. This will be sigma star 2 s if we see the orbital or if we are in it. Finally, we need to draw the mo electron diagram. In o 2 and f 2, there is a crossover of the sigma and the pi ortbials: Web to draw the mo energy diagram for a period 2 homonuclear diatomic molecule, one needs to understand the principles of mo theory, such as energy levels, atomic orbital overlaps, and electron configurations, especially. The berylium 2 molecule has a negative charge, so we need to draw a diagram for it. Add the energies together to get the total energy for the molecule. Web if you have two atomic orbitals, you need two molecular orbitals. Web chemical bonding drawing the mo energy diagram for a period 2 homodiatom draw the molecular orbital (mo) electron. In o 2 and f 2, there is a crossover of the sigma and the pi ortbials: I'm not sure where to start. We use the diagram in part (a) in figure 9.8.1; Draw the mo energy diagram. Be sure your diagram contains all of the electrons in the ion, including any core electrons. We need to draw a diagram of the berylium 2 molecule having a negative charge. We use the diagram in part (a) in figure 9.8.1; Then you fill those lines (molecular orbitals) with 4 electrons (two from each beryllium) there’s you’re mo diagram. 9.6k views 2 years ago. Information from the mo diagram justify o2's stability and show that it's. Be sure your diagram contains all of the electrons in the ion, including any core electrons. Information from the mo diagram justify o2's stability and show that it's bonding order is 2. Draw the mo energy diagram. Draw the energy levels for each orbital. We need to draw a diagram of the berylium 2 molecule having a negative charge. At last sigma 2 pitso, 2 will be further proceeded by sigma 2 and i begin. Finally, we need to draw the mo electron diagram. 9.6k views 2 years ago. Draw the mo energy diagram. I'm not sure where to start. Web drawing the mo energy diagram for a period 2 homodiatom draw the molecular orbital (mo) electron diagram for the f2 molecular ion. Draw the energy levels for each orbital. Be sure your diagram contains all of the electrons in the molecule, including any core electrons. The relative energies of the sigma orbitals drop below that of the pi orbitals'.. The lowest one is your bonding, the higher one is your antibonding. We need to draw a diagram of the berylium 2 molecule having a negative charge. Draw the mo energy diagram. Finally, we need to draw the mo electron diagram. Web how do i approach in drawing the mo energy diagram for a period 2 homodiatom? O chemical bonding drawing the mo energy diagram for a period 2 homodiatom draw the molecular orbital (mo) electron diagram for the be2 molecular ion. The relative energies of the sigma orbitals drop below that of the pi orbitals'. Web to draw the mo energy diagram for a period 2 homonuclear diatomic molecule, one needs to understand the principles of. Web if you have two atomic orbitals, you need two molecular orbitals. Web how do i approach in drawing the mo energy diagram for a period 2 homodiatom? Draw the molecular orbital electron diagram. Be sure your diagram contains all of the electrons in the ion, including any core electrons. The energy of the 02 molecular ion is shown in. I'm not sure where to start. At last sigma 2 pitso, 2 will be further proceeded by sigma 2 and i begin. The berylium 2 molecule has a negative charge, so we need to draw a diagram for it. This will be sigma star 2 s, if we see the orbital or if we are in it. The berylium 2 molecule has a negative charge and we need to draw a diagram for it. Be sure your diagram contains all of the electrons in the molecule, including any core electrons. The increasing order of orbitals Then you fill those lines (molecular orbitals) with 4 electrons (two from each beryllium) there’s you’re mo diagram. Web drawing the mo energy diagram for a period 2 homodiatom draw the molecular orbital (mo) electron diagram for the f2 molecular ion. Web draw the molecular orbital (mo) electron diagram for the b 2 2 − molecular ion. Be sure your diagram contains all of the electrons in the ion, including any core electrons. Web describes the energy diagram for mo from 2nd period elements and the difference in certain mo depending on the type of atomic orbitals used in the linear combination. We need to draw a diagram of the berylium 2 molecule having a negative charge. Be sure your diagram contains all of the electrons in the ion, including any core electrons. This will be the sigma star 2 s if we see the orbital or if we're in it. Draw the energy levels for each orbital.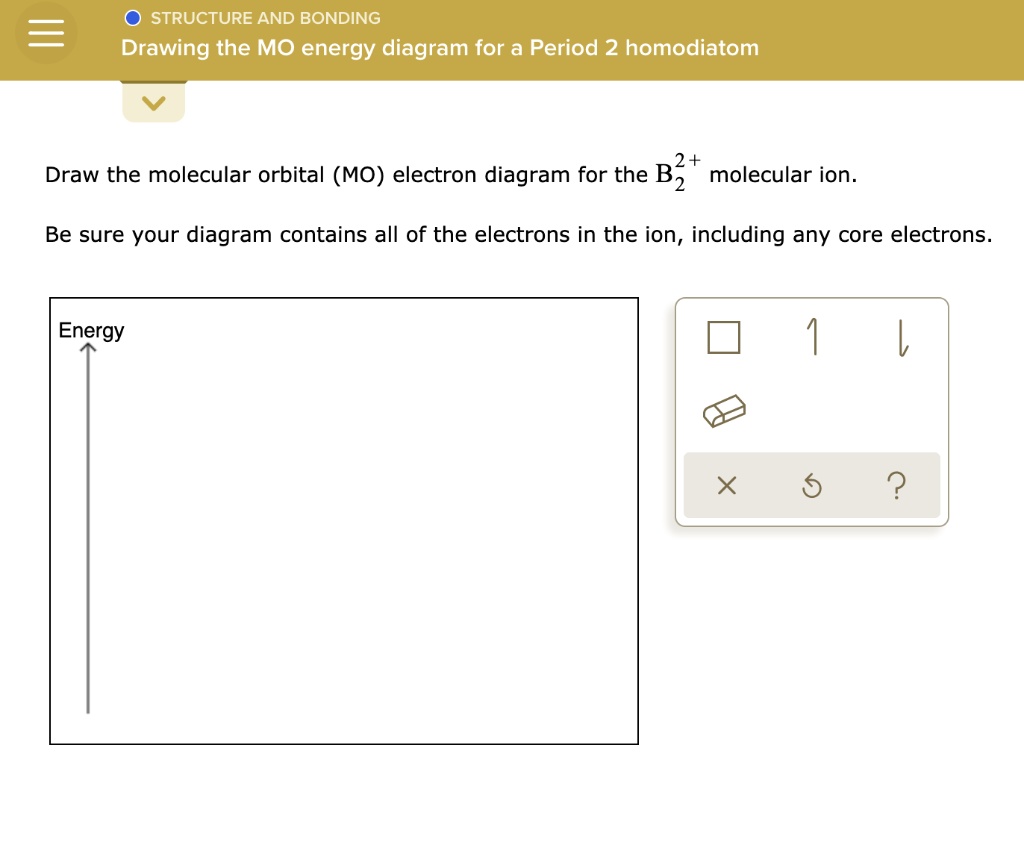
SOLVED STRUCTURE AND BONDING Drawing the MO energy diagram for a

Drawing the MO energy diagram for a Period 2 homodiatom YouTube

ALEKS Drawing the MO energy diagram for a Period 2 homodiatom YouTube

Aleks Drawing the MO energy diagram for a period 2 homodiatom YouTube
SOLVED STRUCTURE AND BONDING Drawing the MO energy diagram for a
CHEMICAL BONDINGDrawing the MO energy diagram for a P… SolvedLib
Solved CHEMICAL BONDING Drawing the MO energy diagram for a
MO Diagrams for First Row Diatomic Molecules Chemistry LibreTexts

9.3e Drawing the MO energy diagram for a Period 2 homodiatom YouTube

Drawing the MO energy diagram for a Period 2 homodiatom YouTube
Finally, We Need To Draw The Mo Electron Diagram.
The Lowest One Is Your Bonding, The Higher One Is Your Antibonding.
Information From The Mo Diagram Justify O2'S Stability And Show That It's Bonding Order Is 2.
So We Are Draw Drawing That Toupee And Two With Structure That Energy.
Related Post: