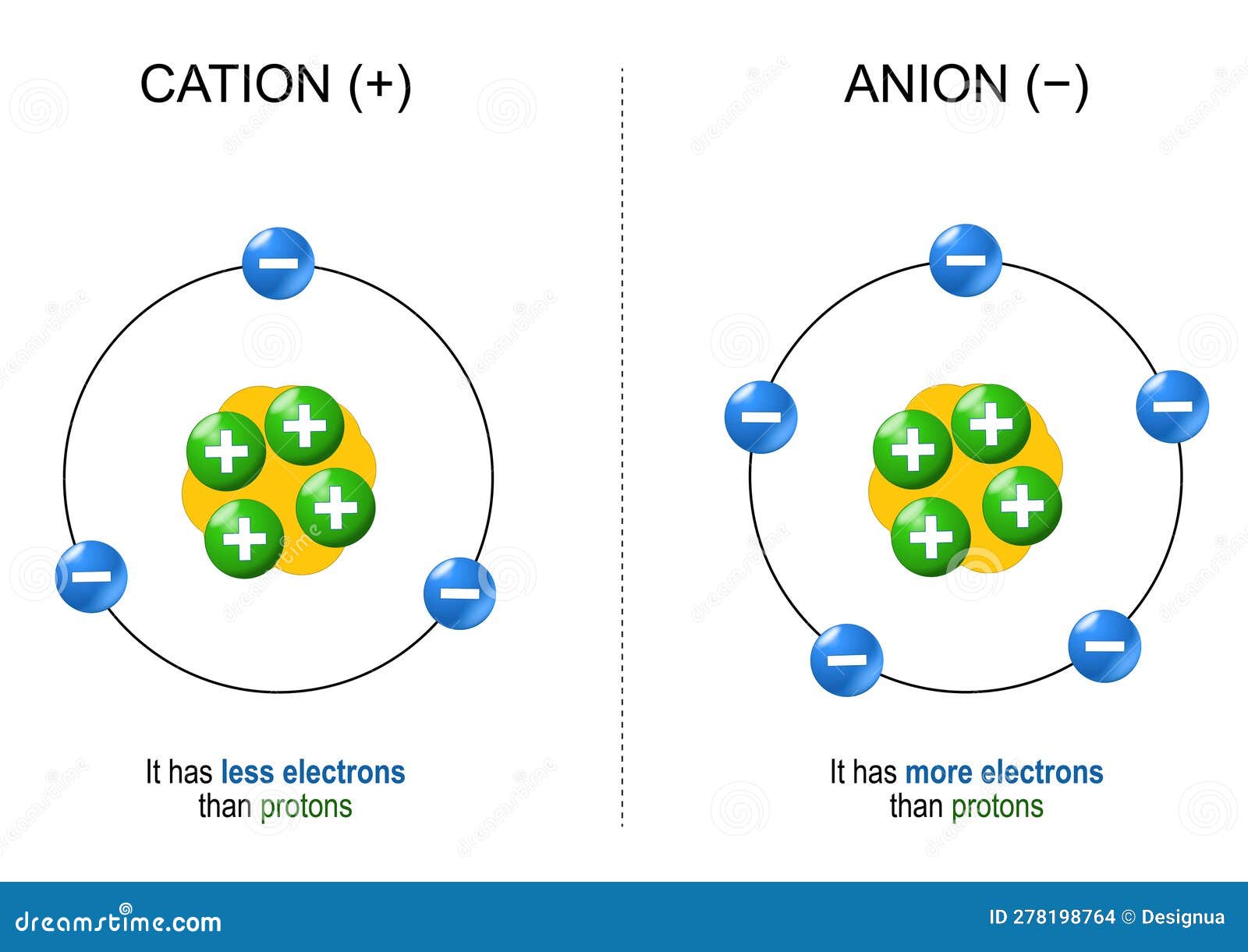
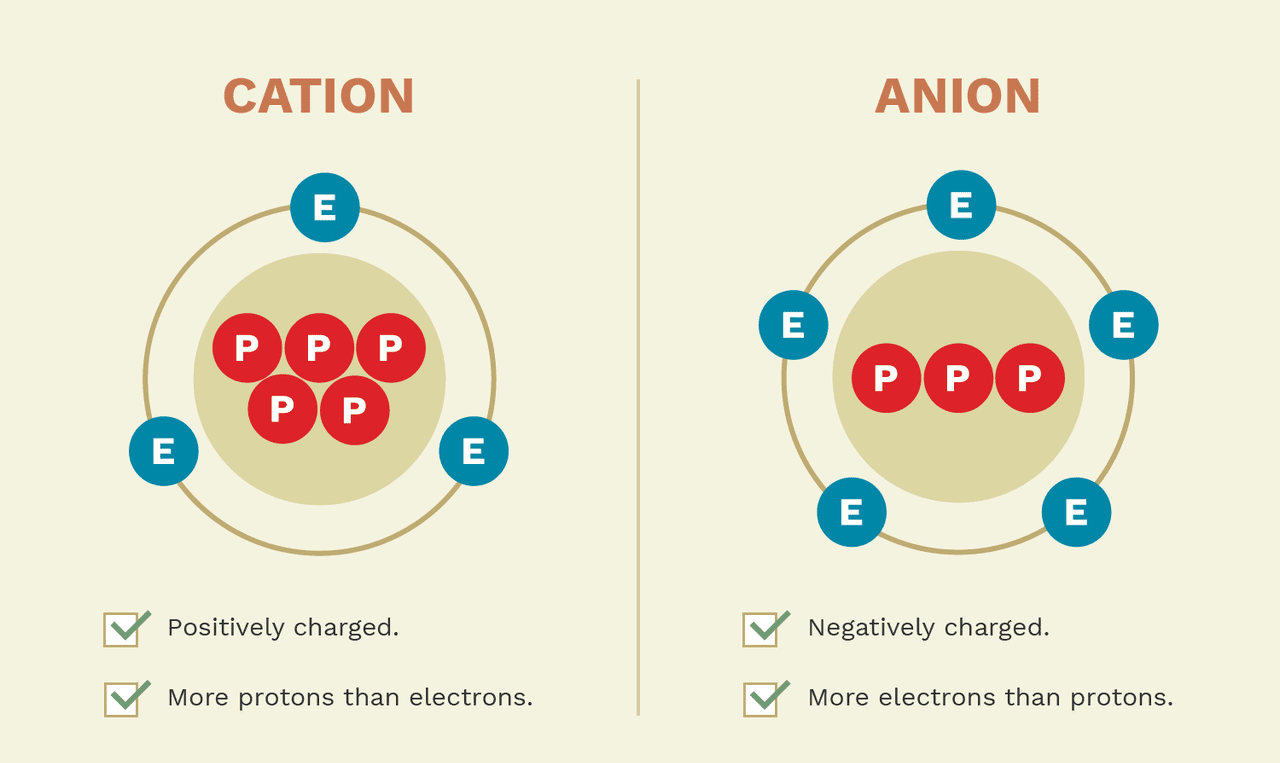
Drawing Of Cations, In section 4.7, we demonstrated that ions are formed by losing electrons to make cations, or by gaining electrons to form anions.
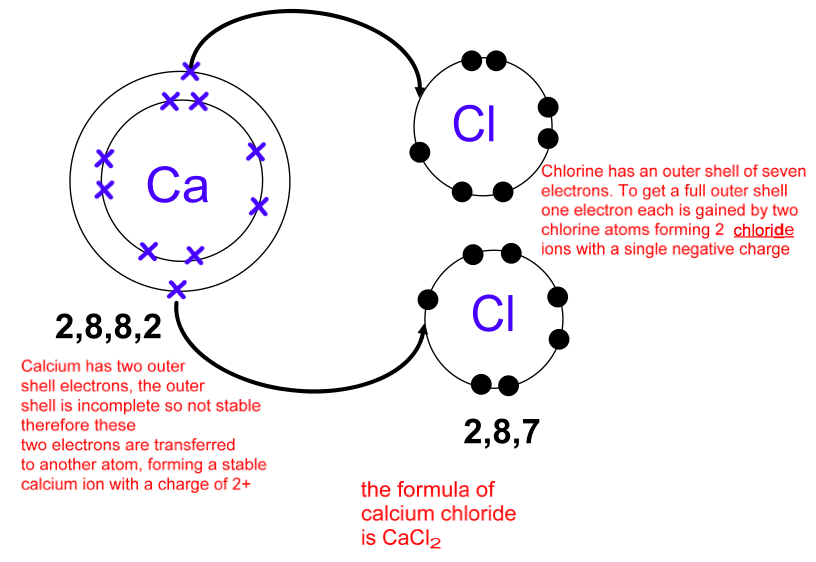
Drawing Of Cations - The astute reader may have noticed something: Indicate the direction of flow for each of the following in a cell illustration where the anode was placed on the right and the cathode on the left. Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms of hyperconjugation and inductive effects. The structure of an ionic solid can be represented using a particulate model. Gesture drawing is a great foundation skill, but of course there is so much more to becoming a capable artist. The total number of electrons does not change. The crystal structure of sodium chloride, \(\ce{nacl}\), a typical ionic compound. The word “cation” comes from the greek word ánō, which means “up.” examples of cations include: The process of finding out what compounds are contained in a sample is called. Draw a point in the intersection of the perpendicular lines: The word cation comes from the greek word kato, which means down. a cation has more protons than electrons, giving it a net positive charge. The crystal structure of sodium chloride, \(\ce{nacl}\), a typical ionic compound. In section 4.7, we demonstrated that ions are formed by losing electrons to make cations, or by gaining electrons to form anions. The smaller. Writing lewis dot symbols of elements. The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\). The process of finding out how much of a compound is contained in a sample is called quantitative analysis. The structure of an ionic solid can be represented using a particulate model. Draw a perpendicular line toward. For anions, add one electron for each negative charge. The process of finding out what compounds are contained in a sample is called. Web a complete guide on how to draw the molecular orbitals of the allyl cation, allyl radical, and the allyl anion, including where to put the nodes. A positively charged ion (a cation) has more protons than. Cations are formed when atoms lose electrons, represented by fewer lewis dots, whereas anions are formed by atoms gaining electrons. Want to join the conversation? What is the lewis electron dot symbol for each element? Web draw lewis structures for ionic compounds. A negatively charged ion (an anion) has more electrons than protons. The flow chart shown below shows the summary of the separation of common cations in. Web the cations (cu +1 and hg +2) fill the tetrahedral holes in two adjacent cubic cells to provide a neutral compound with the correct molecular formula (cu 2 hgi 4). The total number of electrons does not change. Web describe the geometry of a. A positively charged ion (a cation) has more protons than electrons. Web principles of the qualitative analysis of cations. Gesture drawing is a great foundation skill, but of course there is so much more to becoming a capable artist. Web cations are formed when atoms lose electrons, represented by fewer lewis dots, whereas anions are formed by atoms gaining electrons.. The process of finding out what compounds are contained in a sample is called. For anions, add one electron for each negative charge. The flow chart shown below shows the summary of the separation of common cations in. The total number of electrons does not change. Cations are formed when atoms lose electrons, represented by fewer lewis dots, whereas anions. Web draw lewis structures for ionic compounds. Writing lewis dot symbols of elements. The flow of electrons b. The total number of electrons does not change. The process of finding out how much of a compound is contained in a sample is called quantitative analysis. Web draw lewis structures for ionic compounds. Arrange a given series of carbocations in order of increasing or decreasing stability. Web the cations (cu +1 and hg +2) fill the tetrahedral holes in two adjacent cubic cells to provide a neutral compound with the correct molecular formula (cu 2 hgi 4). The systematic analysis of cations is an integral part. If you haven't mastered the art of drawing lewis structures yet, you can check out. Indicate the direction of flow for each of the following in a cell illustration where the anode was placed on the right and the cathode on the left. A positively charged ion (a cation) has more protons than electrons. The word cation comes from the. The flow chart shown below shows the summary of the separation of common cations in. Web the cations and anions in an ionic solid are arranged in a lattice structure that maximizes the attractive forces between opposite charges and minimizes the repulsive forces between like charges. The systematic analysis of cations is an integral part of the salt analysis (or systematic qualitative analysis). For example, a cation with a +2 charge is a dication. Cations with multiple charges may be given special names. Arrange a given series of carbocations in order of increasing or decreasing stability. Web for cations, subtract one electron for each positive charge. Why do the cations move, rather than the ions? Indicate the direction of flow for each of the following in a cell illustration where the anode was placed on the right and the cathode on the left. The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\). One with a +3 charge is a. Web a cation is an ionic species with a positive charge. The astute reader may have noticed something: The trick is called “frost circles”, or, sometimes, the “polygon method”. Gesture drawing is a great foundation skill, but of course there is so much more to becoming a capable artist. Web draw lewis structures for ionic compounds.
Cations and Anions Definitions, Examples, and Differences

Cation Ap Chemistry, Protons, Anatomy And Physiology, Sodium, Physics
Cations and Anions. Structure of Ions Stock Vector Illustration of
Ejemplos De Cationes Quimica Image to u
ALevel Chemistry 1.6.1c draw electron configuration diagrams of

Ortep drawing of the molecular structure of cations in (a

Spacefilling drawing of cations (A) and anions (B) of... Download

Chemical structure of cations and anions commonly used to form ionic
Basic Chemistry Ions, Cations, and Anions
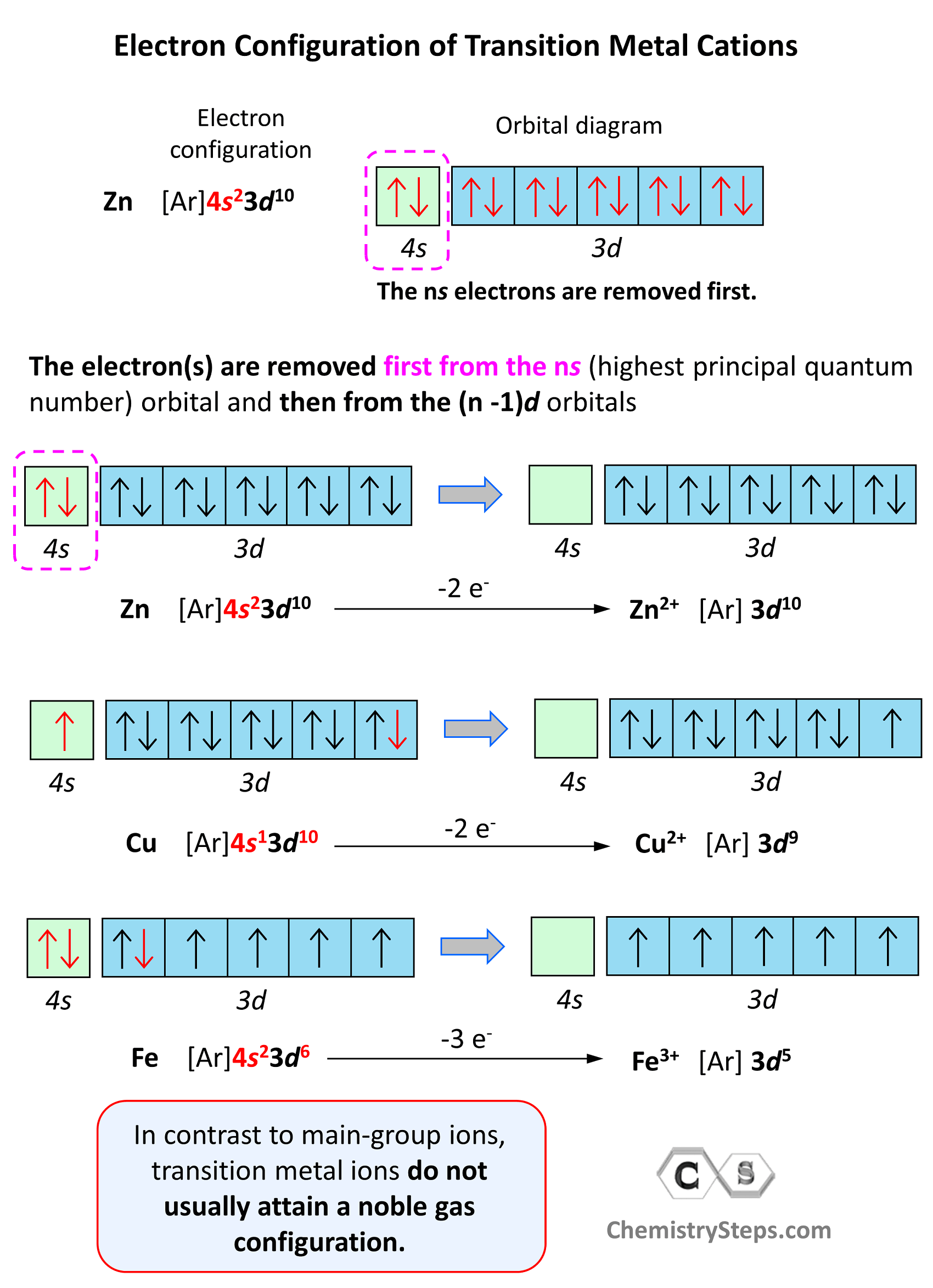
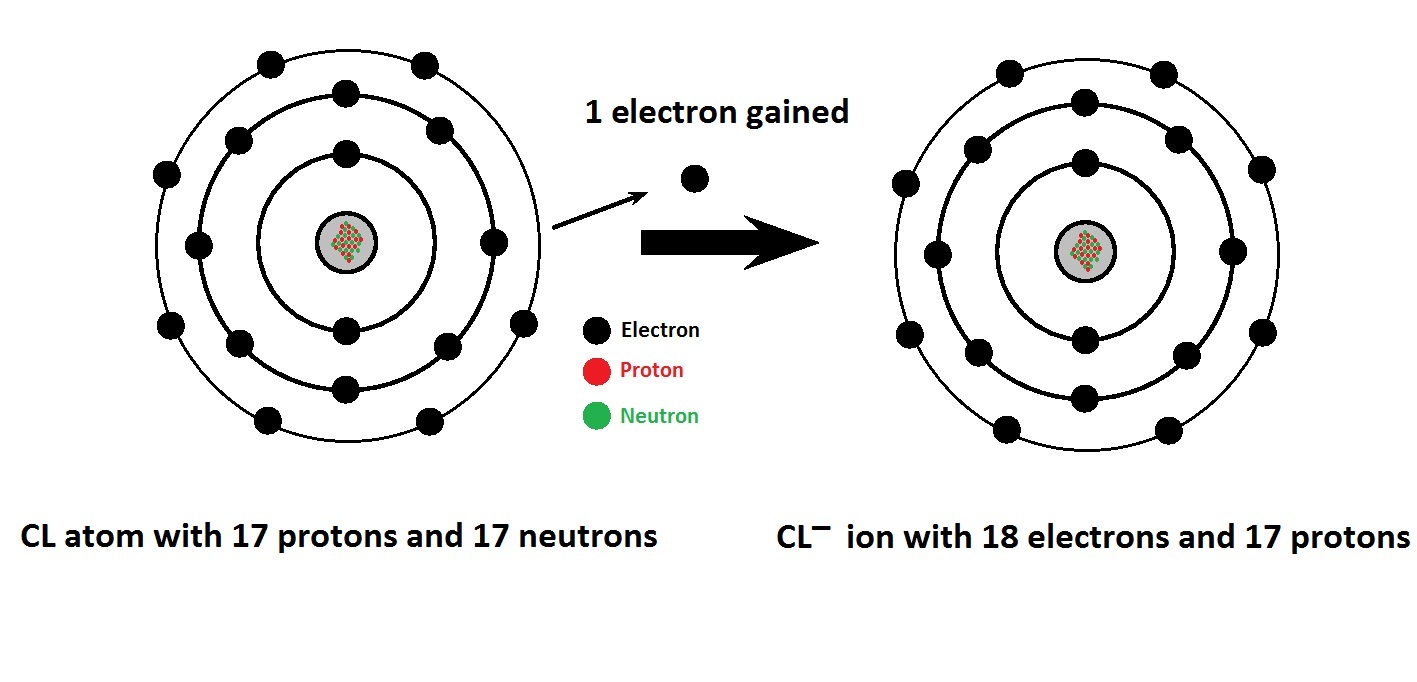
Electron Configurations of Ions Chemistry Steps
Cations Are Formed When Atoms Lose Electrons, Represented By Fewer Lewis Dots, Whereas Anions Are Formed By Atoms Gaining Electrons.
Web The Separation Of Cations In Groups, Along With The Separation Of Ions Within A Group And Their Confirmation Tests Are Described In Detail In Later Chapters.
An Atom With An Electric Charge Is Called An Ion.
Because An Electron Is Removed To Form A Cation, The Cation Of An Atom Can Be Smaller Than The Neutral Atom.
Related Post: