Drawing D Orbitals, 2) always shade your orbitals appropriately to represent the signs of the wave function.
Drawing D Orbitals - They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom. Web you can puzzle them out from the rotating images, the dot density diagrams and the orbital surface diagrams if you like, but analysis of these orbitals is usually considered beyond the scope of general chemistry. Orbital diagrams must follow 3 rules: Web in three of the d orbitals, the lobes of electron density are oriented between the x and y, x and z, and y and z planes; Starting with the third principle quantum number d orbitals form, and there are 5 of these. Orbital diagrams are a visual way to show where the electrons are located within an atom. Δ δ bonds can form between two d d orbitals with appropriate symmetry. Web creating molecular orbital diagrams for molecules with more than two atoms relies on the same basic ideas as the diatomic examples presented here. 2.8k views 8 months ago physical chemistry. For example, when two atoms bond along the z z axis, the dxy d x y orbitals and the two dx2−y2 d x 2 − y 2 orbitals can form δ δ bonds (figure. Web steps for drawing an orbital diagram. This is the \(3d_{x^2−y^2}\) orbital. Web is the elctron subshell the s, p, d and f orbitals? Remember, we can use the periodic table to help us. Web this demonstration shows the basic characteristics for a chosen set of 16 atomic orbitals: And is it a probability function describing where an electron is likely to be? You should, however, be aware that there are five possible orientations for d orbitals. Web #seanchuachemistry #h2chemistry #alevelchemistry in this video, learn about the meaning and shapes of atomic orbitals, namely s, p and d orbitals. Web electronic structure of atoms. How to draw d orbitals? Once again we are looking at ones that are defined by the cartesian coordinate system. Web is the elctron subshell the s, p, d and f orbitals? An #s# orbital is a sphere. It explores s and p orbitals in some detail, including their shapes and energies. How can one* assemble* all four quantum number and get the orbitals, subshells. However, with more atoms, computers are required to calculate how the atomic orbitals combine. Web this page discusses atomic orbitals at an introductory level. You will note that the 3 d orbits have two nodal surfaces. Web you can puzzle them out from the rotating images, the dot density diagrams and the orbital surface diagrams if you like, but analysis. Web steps for drawing an orbital diagram. Remember, we can use the periodic table to help us. The type, the absolute value of quantum number , the number of lobes/nodes, the cartesian polynomial form of the wavefunctions, and two 3d views of the probability density (boundary surface: Web interactive colour surface representations for the five d orbitals in 3d showing. D orbitals are described only in terms of their energy, and f orbitals are only mentioned in passing. Web 1) draw each orbital superimposed on a labeled coordinate system (i.e. Web 230313 how to draw shapes of orbitals. Draw a long vertical arrow that points upward. How can one* assemble* all four quantum number and get the orbitals, subshells structures? Remember, we can use the periodic table to help us. In orbitals diagrams, the orbitals are shown as boxes, and the electrons in them as arrows pointing up or down. Web interactive colour surface representations for the five d orbitals in 3d showing the nodes important for transition metal chemistry. They offer a way to calculate the probability of finding. Web 230313 how to draw shapes of orbitals. These orbitals are referred to as the \(3d_{xy}\), \)3d_{xz}\), and \(3d_{yz}\) orbitals, respectively. Axes and labels can be. How to draw d orbitals? A fourth d orbital has lobes lying along the x and y axes; D orbitals are described only in terms of their energy, and f orbitals are only mentioned in passing. How to draw d orbitals? Orbital diagrams must follow 3 rules: Web creating molecular orbital diagrams for molecules with more than two atoms relies on the same basic ideas as the diatomic examples presented here. A fourth d orbital has lobes lying. Web this page discusses atomic orbitals at an introductory level. In orbitals diagrams, the orbitals are shown as boxes, and the electrons in them as arrows pointing up or down. Starting with the third principle quantum number d orbitals form, and there are 5 of these. For example, when two atoms bond along the z z axis, the dxy d. However, with more atoms, computers are required to calculate how the atomic orbitals combine. For example, when two atoms bond along the z z axis, the dxy d x y orbitals and the two dx2−y2 d x 2 − y 2 orbitals can form δ δ bonds (figure. Web interactive colour surface representations for the five d orbitals in 3d showing the nodes important for transition metal chemistry. You will note that the 3 d orbits have two nodal surfaces. Learn all of this in this lesson with examples at the end. Web this page discusses atomic orbitals at an introductory level. Web is the elctron subshell the s, p, d and f orbitals? Draw the x, y, z axes first and then draw your orbital on top of the axis set). Web for an #s# orbital, draw a circle; Δ δ bonds can form between two d d orbitals with appropriate symmetry. Each box represents one orbital, and each arrow indicates one electron. You should, however, be aware that there are five possible orientations for d orbitals. How can one imagine the structure of a real atom not one which bohr model suggests but an original one observed by solving the wave equation. Write out the electron configuration to determine which orbitals are filled. This lesson particularly revolves around o and a level. Web in three of the d orbitals, the lobes of electron density are oriented between the x and y, x and z, and y and z planes;
how to draw shapes of d orbitals

Chemical bonding Atomic Orbitals, Shapes, Hybridization Britannica
Drawing Molecular Orbital Diagrams

How To Draw Shapes Of D Orbitals at How To Draw

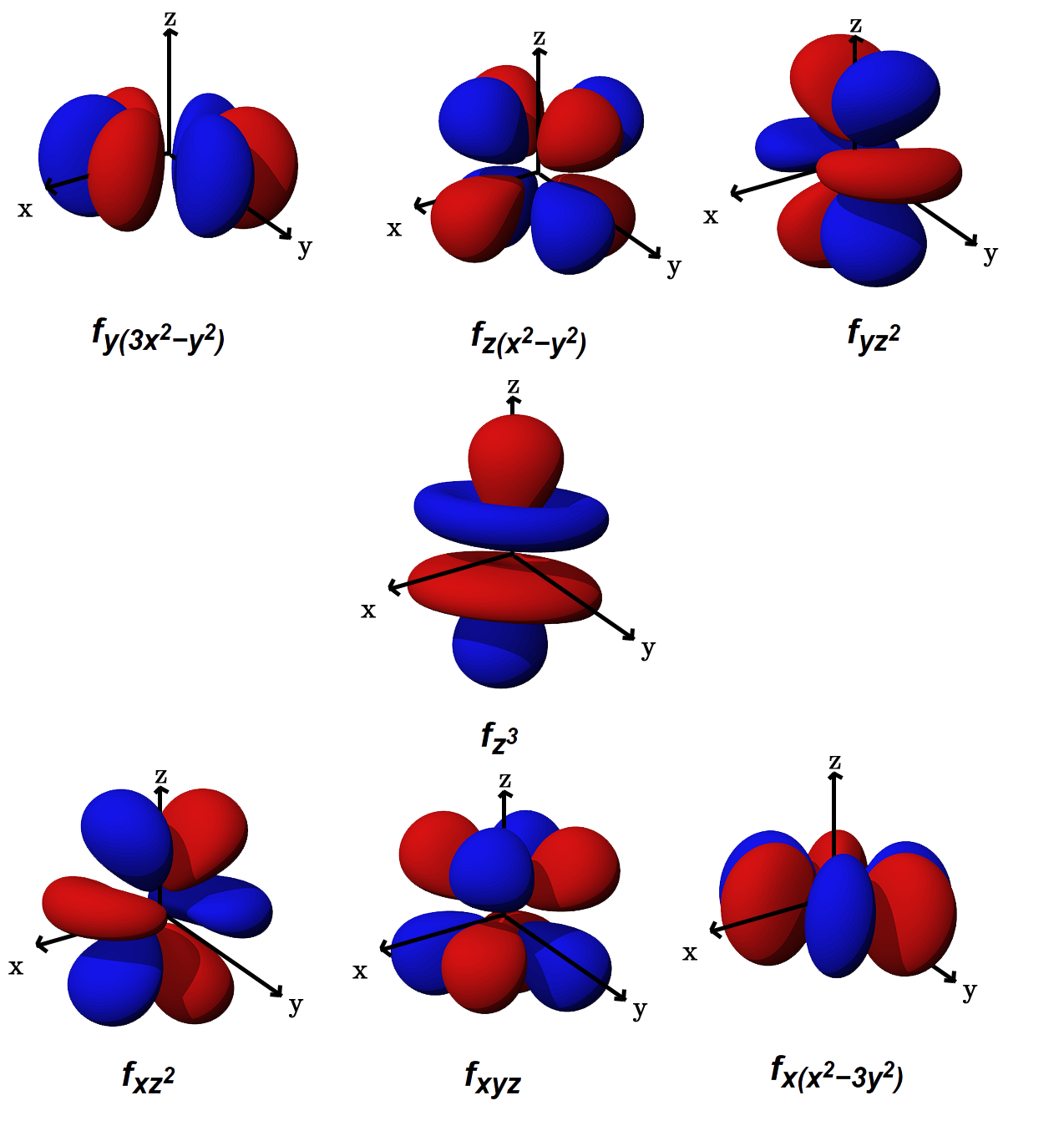
Five D Orbitals

How to Draw Shapes of D Orbitals Bell Evaithere

how to draw shapes of d orbitals elliottlyde
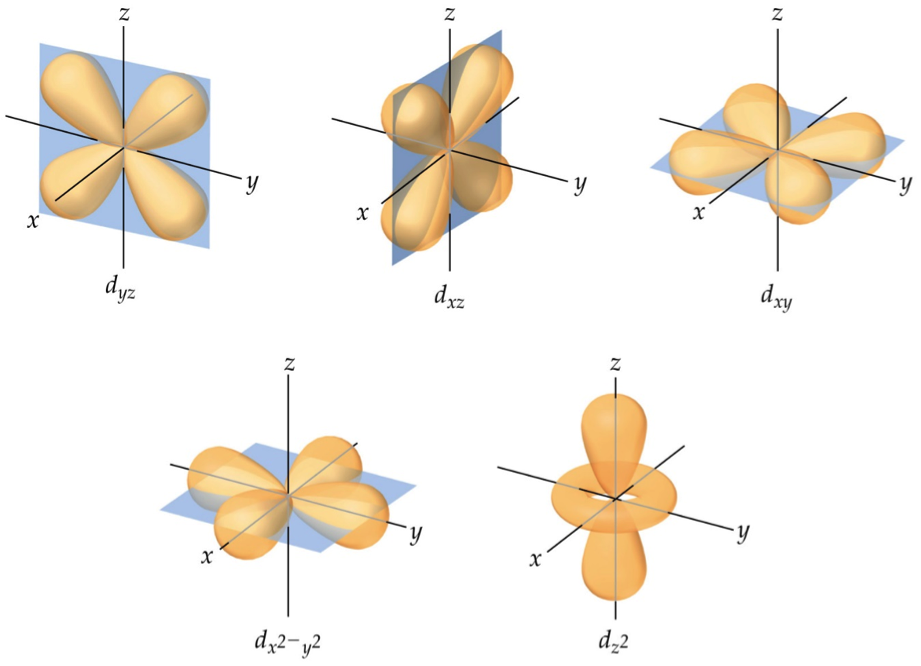
The Shapes of Atomic Orbitals Chemistry

Atomic orbitals explained polizhuge

How to Draw Shapes of Orbitals
2.8K Views 8 Months Ago Physical Chemistry.
Web Draw The Shapes Of Various `P` And `D` Orbitals.
This Is A Way Of.
Below Are Representations Of The D.
Related Post: