Draw The Lewis Structure For The Nf3, Web lewis structure of nf 3 can be drawn by starting from valence electrons of nitrogen and fluorine atoms in several steps.
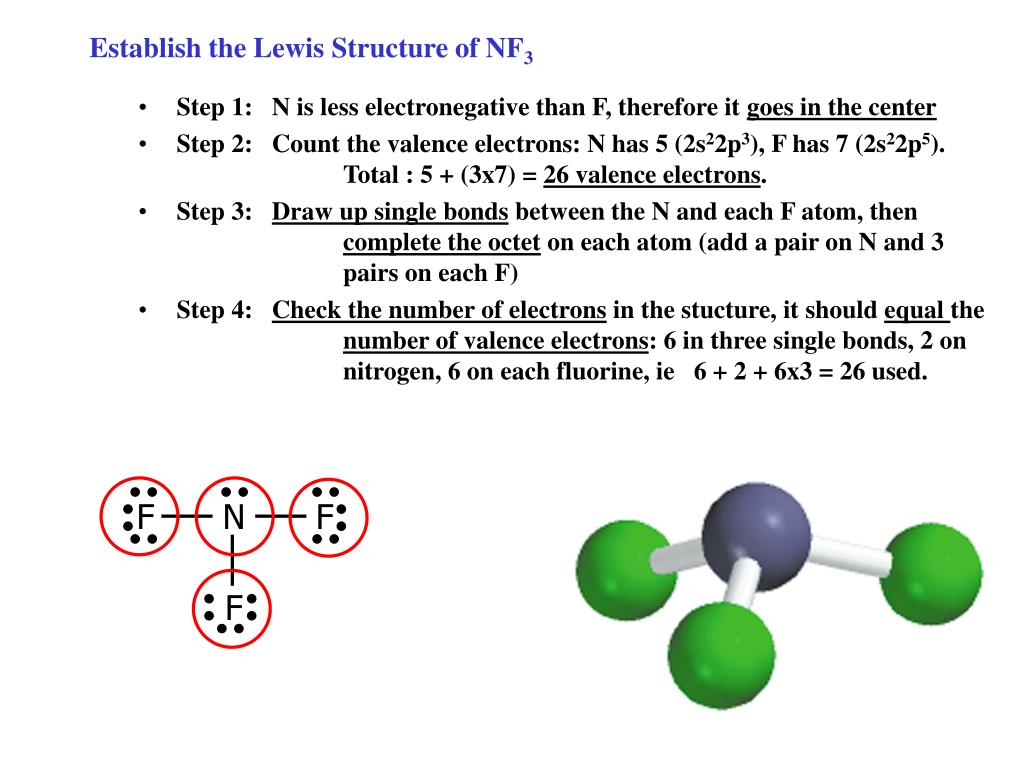
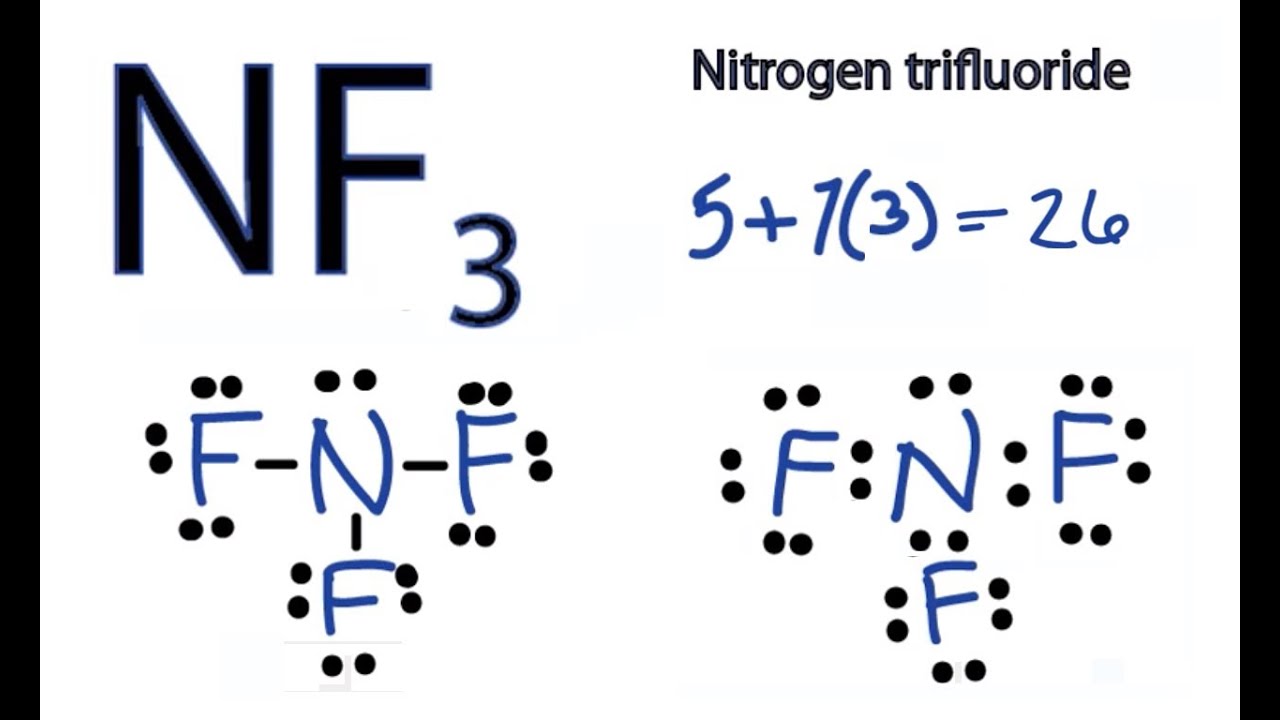
Draw The Lewis Structure For The Nf3 - #4 calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. After drawing the lewis structure of nf 3, you can decide shape of the nf 3 molecule. There are 3 single bonds between the nitrogen atom (n) and each fluorine atom (f). Web drawing lewis structures for molecules with one central atom: Its noticeable characteristics include being colorless and carrying. It consists of one nitrogen and three fluorine atoms. Begin by identifying the number of valence electrons for each atom in the nf3 molecule. Web lewis structure of nf3 contains three single bonds between the nitrogen (n) atom and each fluorine (f) atom. And then fluorine is in group 7 or 17, it has 7. This information is important in determining the chemical properties and reactivity of nf3. On the periodic table, nitrogen is in group 5 or 15, so it has 5 valence electrons; Web drawing the lewis structure of nf 3 involves several steps starting from the valence electrons of nitrogen and fluorine atoms, which are explained in detail in this tutorial. And then fluorine is in group 7 or 17, it has 7. By using. Web drawing the lewis structure of nf 3 involves several steps starting from the valence electrons of nitrogen and fluorine atoms, which are explained in detail in this tutorial. Web draw the lewis structure for nf3 (nitrogen is the central atom) and select the correct model representing its electron pair geometry and molecular geometry electron pair geometries linear trigonal planar. Web drawing the lewis structure of nf3. To create the lewis structure of nf3, follow these steps: Web nf3 lewis structure has a nitrogen atom (n) at the center which is surrounded by three fluorine atoms (f). Each step of drawing the lewis structure of nf 3 is explained in detail in this tutorial. Figure out how many electrons the. We first find out the total number of valence. #4 calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. Therefore, the lewis structure of nf contains one lone pair and 3 bond pair. Web we have explained the lewis structure, vsepr theory, hybridization, polarity, and mot. Web here’s how you can easily draw the nf 3 lewis structure step by step: Web next, we draw the nf3 lewis structure by placing the nitrogen atom at the center, bonding three fluorine atoms to it, and adding one lone pair of electrons to the nitrogen atom. Web lewis structure of nf 3 can be drawn by starting from. Web next, we draw the nf3 lewis structure by placing the nitrogen atom at the center, bonding three fluorine atoms to it, and adding one lone pair of electrons to the nitrogen atom. Its noticeable characteristics include being colorless and carrying. Once the lewis structure is drawn, the shape of the nf 3. Web the formation of molecule can easily. Web next, we draw the nf3 lewis structure by placing the nitrogen atom at the center, bonding three fluorine atoms to it, and adding one lone pair of electrons to the nitrogen atom. There is 1 lone pair on the nitrogen atom (n) and 3 lone pairs on all three fluorine atoms (f). Web the lewis dot diagram for nf3. Web next, we draw the nf3 lewis structure by placing the nitrogen atom at the center, bonding three fluorine atoms to it, and adding one lone pair of electrons to the nitrogen atom. Web drawing lewis structures for molecules with one central atom: Each fluorine atom has a lone pair of electrons. Web this chemistry video will show you how. Each step of drawing the lewis structure of nf 3 is explained in detail in this tutorial. There is 1 lone pair on the nitrogen atom (n) and 3 lone pairs on all three fluorine atoms (f). Web the formation of molecule can easily be shown by drawing lewis dot structure. There are 3 single bonds between the nitrogen atom. It shows us the arrangement of the valence electrons and how they are shared between the atoms. Each fluorine atom has a lone pair of electrons. Web the formation of molecule can easily be shown by drawing lewis dot structure. It consists of one nitrogen and three fluorine atoms. Web a video explanation of how to draw the lewis dot. Its noticeable characteristics include being colorless and carrying. Web here’s how you can easily draw the nf 3 lewis structure step by step: Then, we identify four electron domains around the central atom, which is. On the periodic table, nitrogen is in group 5 or 15, so it has 5 valence electrons; Web the lewis dot diagram for nf3 helps us understand the structure and bonding in the molecule. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Web this chemistry video will show you how to draw the lewis structure and determine the molecular geometry for nitrogen trifluoride (nf3). Therefore, the lewis structure of nf contains one lone pair and 3 bond pair. The nitrogen atom has 1 lone pair and all the three fluorine atoms have 3 lone pairs. Web we have explained the lewis structure, vsepr theory, hybridization, polarity, and mot diagram in detail. Each fluorine atom has a lone pair of electrons. There are 3 single bonds between the nitrogen atom (n) and each fluorine atom (f). Begin by identifying the number of valence electrons for each atom in the nf3 molecule. #4 calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. Once the lewis structure is drawn, the shape of the nf 3. The nitrogen atom (n) is at the center and it is surrounded by 3 fluorine atoms (f).
SOLVED Draw the Lewis structure of NF3.
PPT Establish the Lewis Structure of NF 3 PowerPoint Presentation
NF3 Lewis Structure How to Draw the Dot Structure for NF3 (Nitrogen

NF3 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and

So far, we’ve used 26 of the NF3 Lewis structure’s total 26 outermost

Unveiling the Lewis Dot Diagram for NF3 Understanding the Bonding

Lewis electron dot structure for NF3/ How to draw covalent bond for NF3

NF3 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and

NF3 Lewis Structure (Nitrogen Trifluoride) YouTube

Draw The Lewis Structure Of Nf3
Since Atom's Lewis Dot Structure Has Three Dots.
To Create The Lewis Structure Of Nf3, Follow These Steps:
Web Lewis Structure Of Nf 3 Can Be Drawn By Starting From Valence Electrons Of Nitrogen And Fluorine Atoms In Several Steps.
There Is 1 Lone Pair On The Nitrogen Atom (N) And 3 Lone Pairs On All Three Fluorine Atoms (F).
Related Post: