Draw The Lewis Structure For Ccl4, Draw the lewis structure for ccl4.
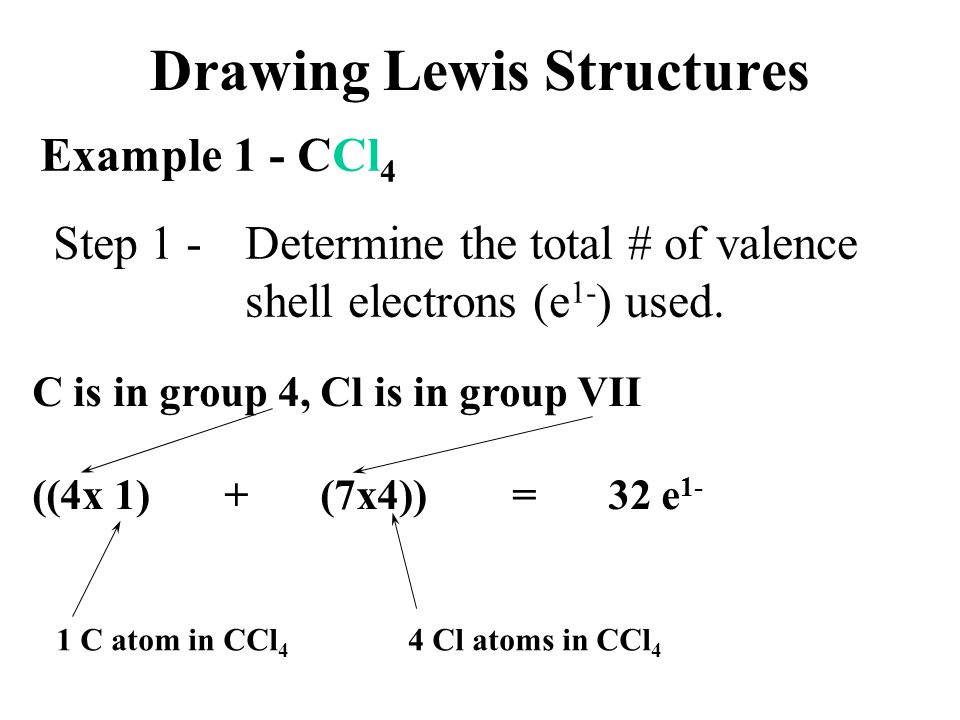
Draw The Lewis Structure For Ccl4 - Begin by determining the total number of valence electrons. Web the lewis structure for carbon tetrachloride (ccl4) involves each of the four chlorines (cl) forming a single bond with the central carbon (c). There are 3 lone pairs on all the four chlorine atoms (cl). Web drawing lewis structures for molecules with one central atom: The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Web in this tutorial, we’ll illustrate the steps to draw the lewis structure for tetrachloromethane, denoted as ccl4. Each step of drawing is explained in detail in this tutorial. Chlorine has 7 valence electrons, but we have 4 chlorines so let's multiply that by 4. Lewis structures are meant to provide a visualization of the atomic structure and the distribution of electrons in a given chemical compound. Web for the lewis structure of ccl4 first, let’s calculate the total valence electrons. Lewis structures are meant to provide a visualization of the atomic structure and the distribution of electrons in a given chemical compound. The following procedure can be used to draw lewis structure for simple molecules. Web draw lewis structures for covalent compounds. That will be the least electronegative atom (#c#). Draw lewis structures depicting the bonding in simple molecules Each step of drawing is explained in detail in this tutorial. Web draw lewis structures for covalent compounds. As there are four molecules of chlorine, we will calculate the number of valence electrons accordingly. Web i quickly take you through how to draw the lewis structure of ccl4 (carbon tetrachloride). Decide which atom is the central atom in the structure. Web i quickly take you through how to draw the lewis structure of ccl4 (carbon tetrachloride). Here are the steps that i follow when drawing a lewis structure. Web in this tutorial, we’ll illustrate the steps to draw the lewis structure for tetrachloromethane, denoted as ccl4. Figure out how many electrons the molecule must have, based on the number of. Find total number of electrons of the valance shells of carbon atom and chlorine atoms; Draw the lewis structure for ccl4. Web a ccl4 lewis structure is a diagram that represents the electron configuration of covalently bonded compounds. Your solution’s ready to go! Web a video explanation of how to draw the lewis dot structure for carbon tetrachloride, along with. Web ccl4 lewis structure has a carbon atom (c) at the center which is surrounded by four chlorine atoms (cl). Web in this tutorial, we’ll illustrate the steps to draw the lewis structure for tetrachloromethane, denoted as ccl4. Draw lewis structures depicting the bonding in simple molecules That will be the least electronegative atom (#c#). Web i quickly take you. Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. Here are the steps that i follow when drawing a lewis structure. That will be the least electronegative atom (#c#). Web a ccl4 lewis structure is a diagram that represents the electron configuration of covalently bonded. Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. Here are the steps that i follow when drawing a lewis structure. Web in this tutorial, we’ll illustrate the steps to draw the lewis structure for tetrachloromethane, denoted as ccl4. Mark lone pairs on atoms Web. Here are the steps that i follow when drawing a lewis structure. Web ccl4 lewis structure has a carbon atom (c) at the center which is surrounded by four chlorine atoms (cl). #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on the atoms, if necessary. So, if you. Web there are several steps to complete the lewis structure of ccl 4. Lewis structure of ccl4 contains four single bonds between the carbon (c) atom and each chlorine (cl) atom. Figure out how many electrons the molecule must have, based on the number of valence electrons in each. Draw lewis structures depicting the bonding in simple molecules This results. Web drawing lewis structures for molecules with one central atom: Each step of drawing is explained in detail in this tutorial. Draw a valid electron dot structure for each of the given elements. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. I also go over hybridization, shape. Web write lewis symbols for neutral atoms and ions; Draw lewis structures for covalent compounds. The lewis structure for ccl4 is a commonly tested lewis struc. Web in this tutorial, we’ll illustrate the steps to draw the lewis structure for tetrachloromethane, denoted as ccl4. Draw the lewis structure for ccl4. The remaining electrons in each chlorine atom form lone. Web for the lewis structure of ccl4 first, let’s calculate the total valence electrons. Web i quickly take you through how to draw the lewis structure of ccl4 (carbon tetrachloride). Begin by determining the total number of valence electrons. Web this is all about the compound ccl4, its lewis structure, hybridization, molecular geometry, polarity, applications, and mo diagram. Mark lone pairs on atoms Find center atom and draw basic skeletal structure; Web there are several steps to complete the lewis structure of ccl 4. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Web the lewis structure for carbon tetrachloride (ccl4) involves each of the four chlorines (cl) forming a single bond with the central carbon (c). Lewis structures are meant to provide a visualization of the atomic structure and the distribution of electrons in a given chemical compound.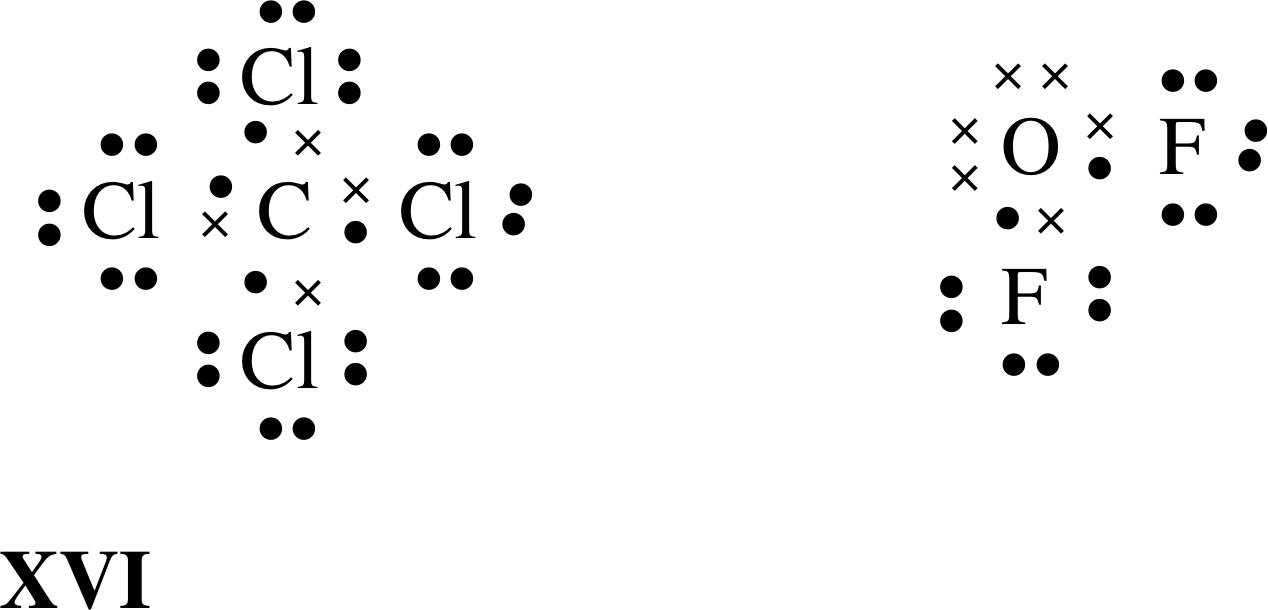
20+ Ccl4 Molecular Geometry Name Full GM

CCl4 Lewis Structure Science Trends
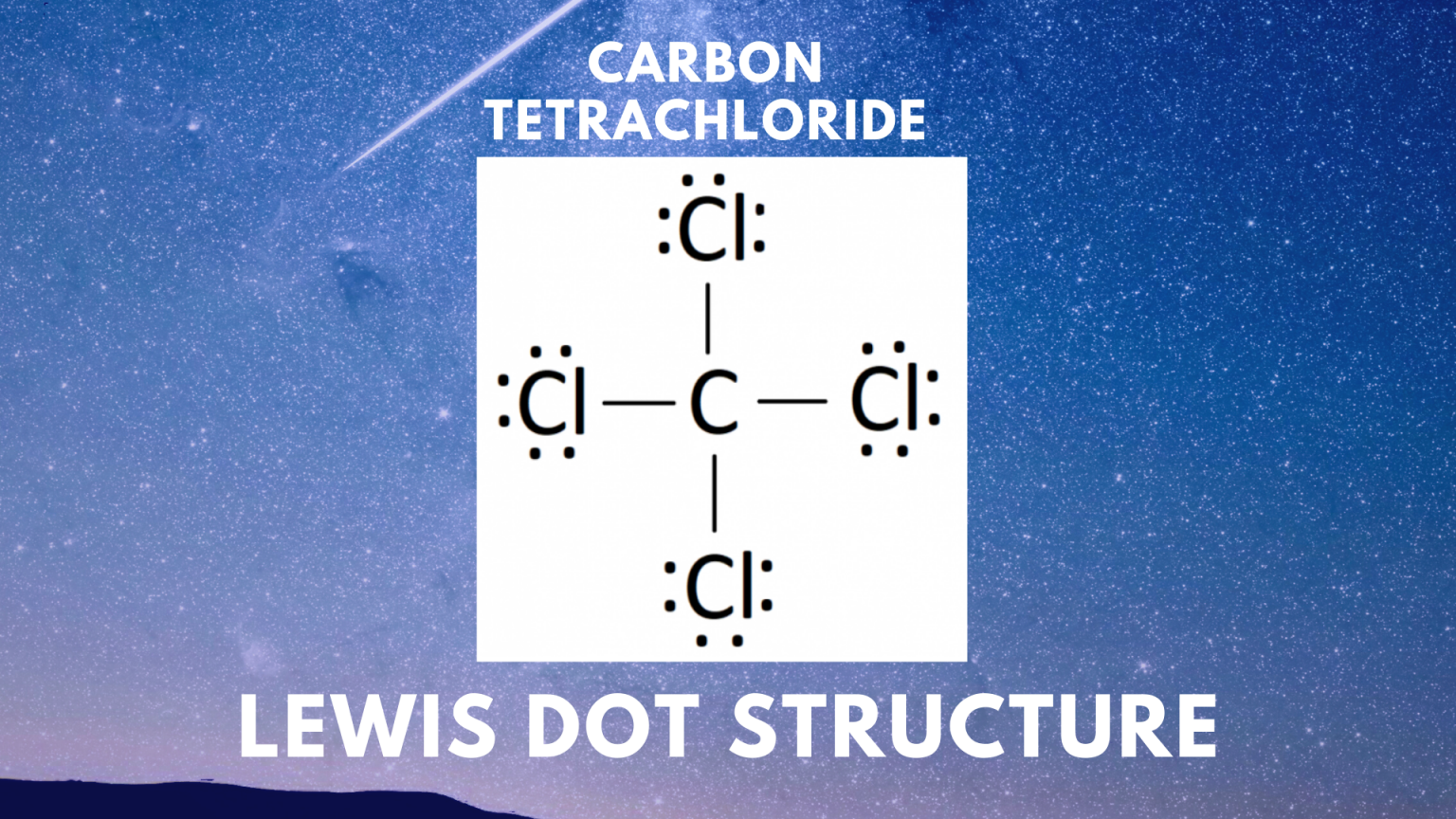
CCl4 Lewis Structure Science Trends
Lewis Dot Diagram For Ccl4 Wiring Site Resource
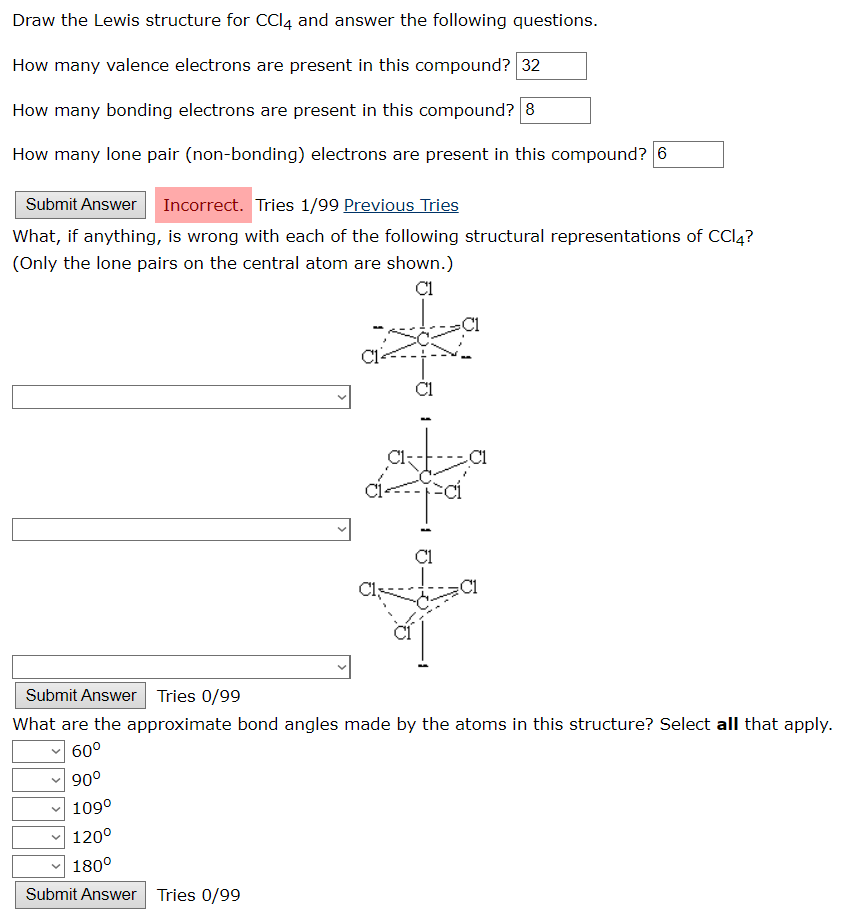
Solved Draw the Lewis structure for CCl4 and answer the

Lewis Structures CCl4 YouTube

Drawing Lewis Structure for CCl4 and Determining Geometries and Bond

CCl4 Lewis Structure ,Valence Electrons ,Formal Charge ,Polar or Nonpolar
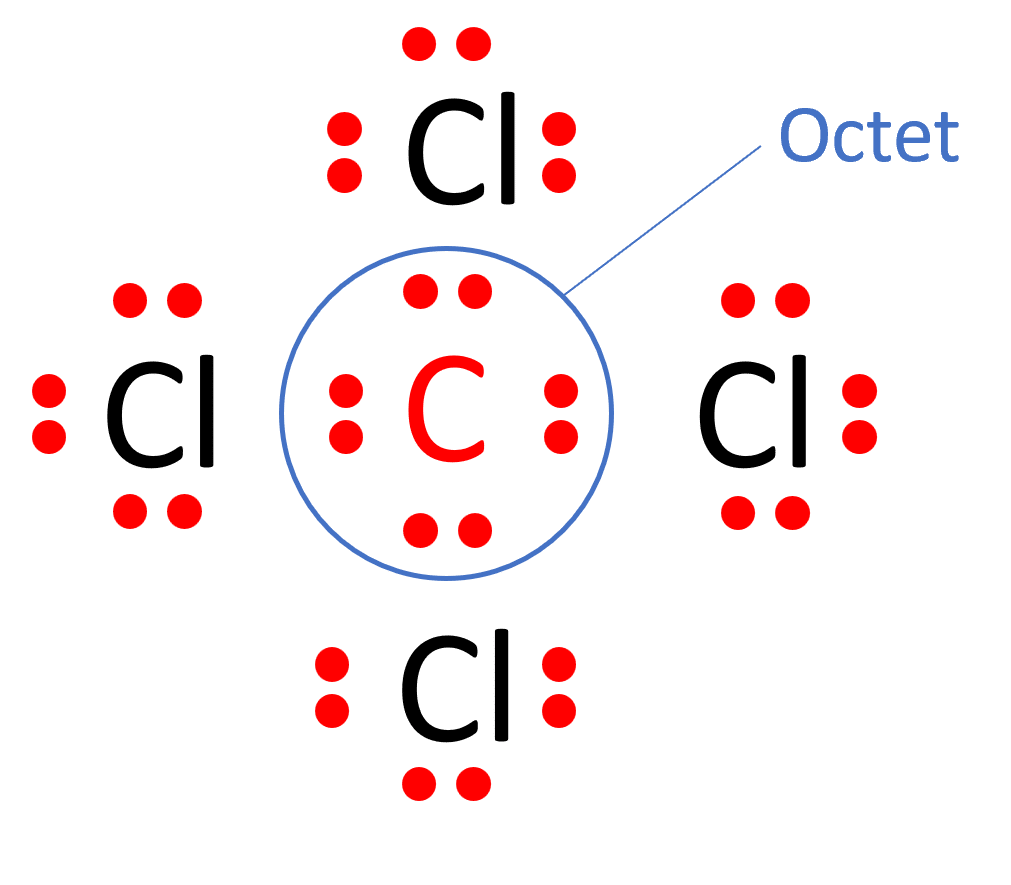
CCl4 Lewis structure in four simple steps What's Insight

CCl4 Lewis Structure (Carbon Tetrachloride) YouTube
Web Ccl4 Lewis Structure Has A Carbon Atom (C) At The Center Which Is Surrounded By Four Chlorine Atoms (Cl).
Carbon Is In Group 4 Or 14, So It Has 4.
Draw The Lewis Structure For Ccl4.
Your Solution’s Ready To Go!
Related Post: