Draw The Lewis Dot Diagram For A H Cation, Assess the stability of a structure by considering formal charges of atoms.
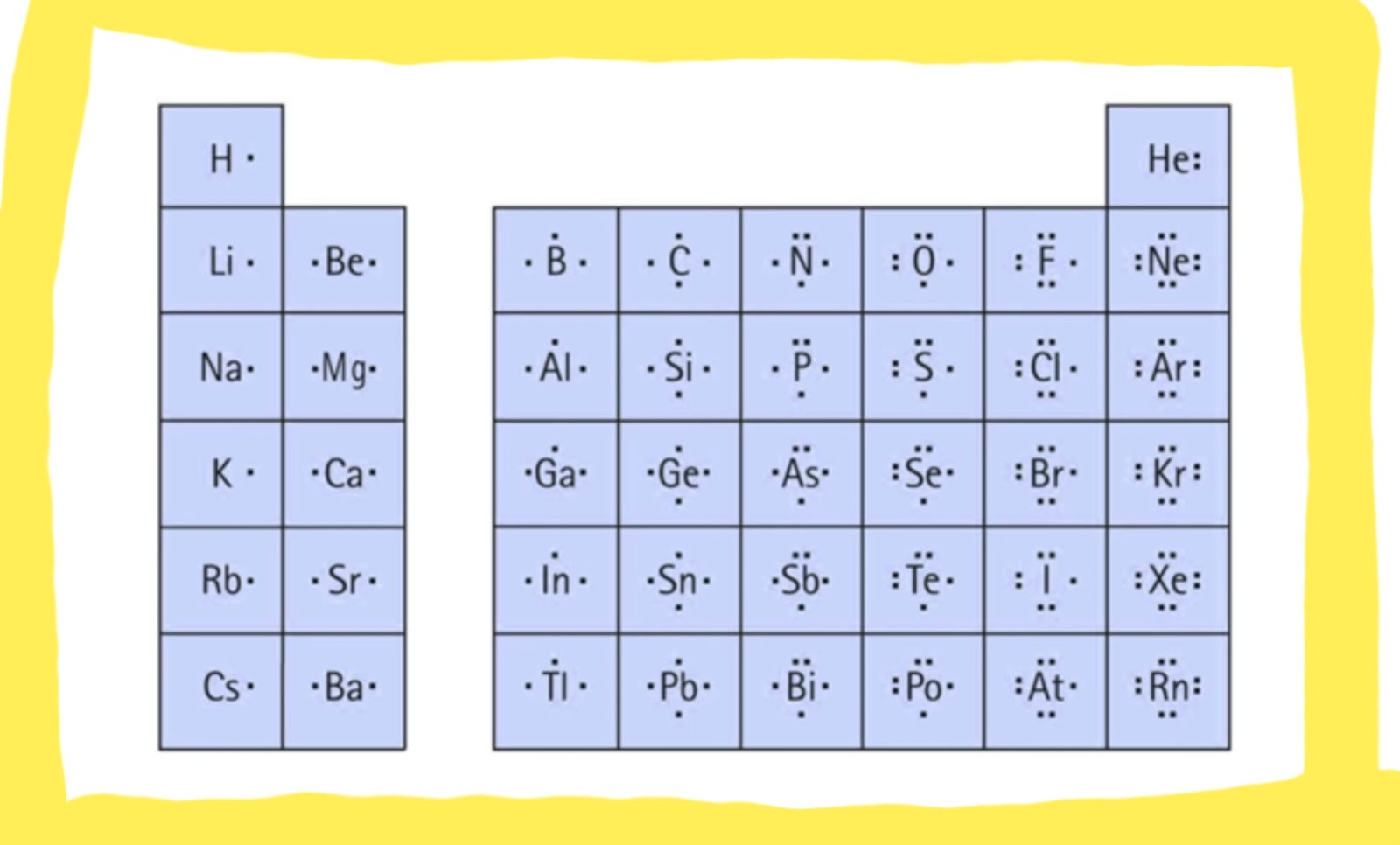
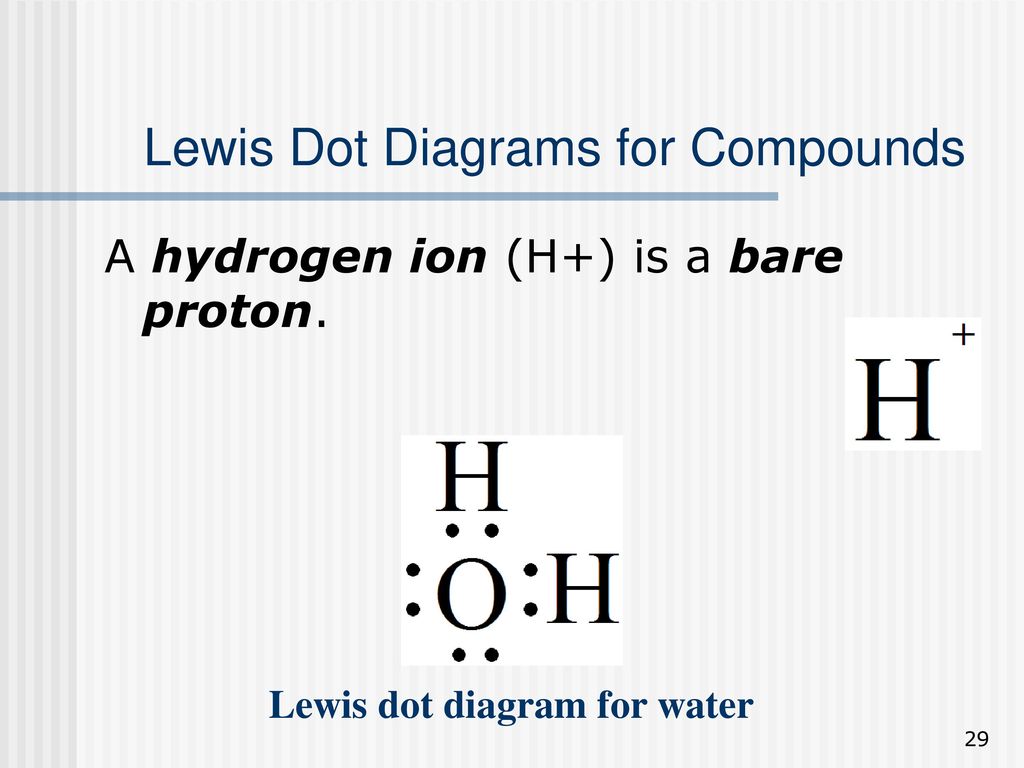
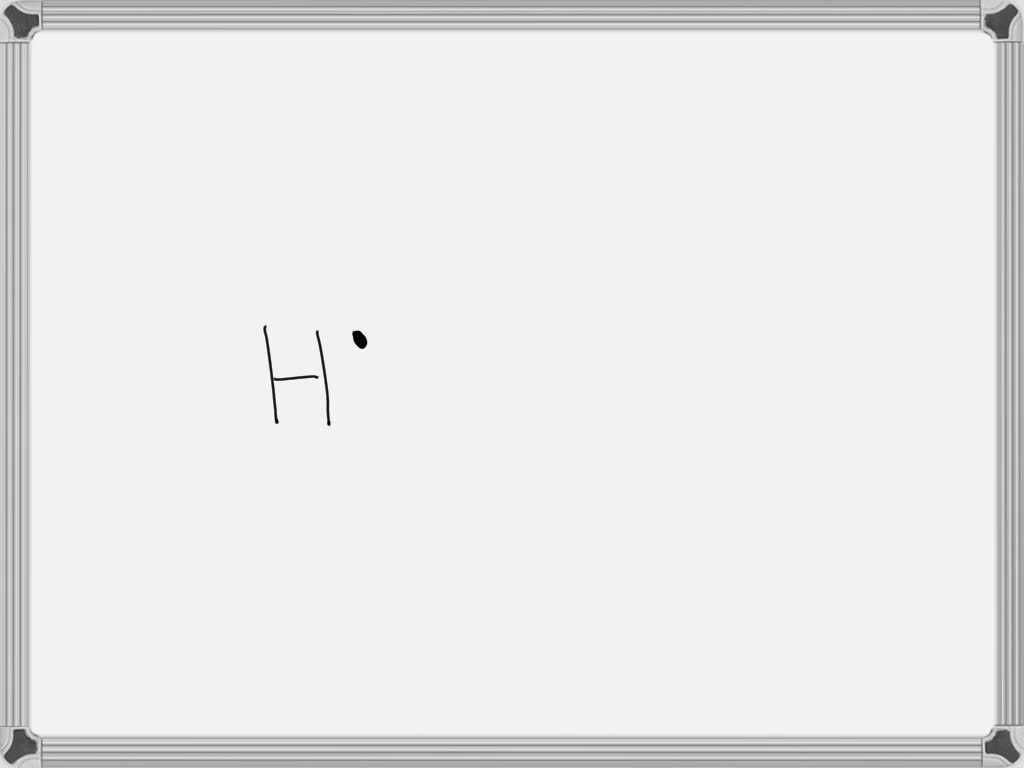
Draw The Lewis Dot Diagram For A H Cation - Give examples for molecules and ions that do not follow the octet rule. Web lewis dot structures can be drawn to show the valence electrons that surround an atom itself. Web the lewis formalism used for the h 2 molecule is h:h or h—h. A lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. Web a lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. A dot is used to represent a valence electron. By the end of this section, you will be able to: The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Web lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Make sure charges are in the diagram. С с c 119 х. 31k views 5 years ago. Web we use lewis symbols to describe valence electron configurations of atoms and monatomic ions. Draw resonance structures of some molecules. Hydrogen follows the doublet rule, so you can pair the. Figure 7.9 shows the lewis symbols for the elements of the third period of the periodic table. Lewis dot structure is the classical bonding model in which only valence electrons of the atoms are used. Draw resonance structures of some molecules. In almost all cases, chemical bonds are formed by. Web a lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom. Web draw the lewis dot diagram for a h+ cation. Web draw the lewis dot. Web ⚛ lewis dot diagrams. 31k views 5 years ago. Valence electrons occupy the highest energy level (also known as the valence shell). There are 2 steps to solve this one. Web the only reasonable lewis electron dot diagram for this compound has the p atom making five covalent bonds: In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. There are 2 steps to solve this one. A lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. This type of lewis dot. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. ⚛ lewis electron dot structures. Assess the stability of a structure by considering formal charges of atoms. See the following examples for how to draw lewis dot structures for common atoms involved in covalent bonding. Assign formal charge to an atom in a dot structure. Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Your solution’s ready to go! Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. A lewis symbol consists of an elemental symbol surrounded by one dot for each of its. A dot is used to represent a valence electron. For the h+ structure use the periodic table to find the total number of valence electrons for the h+ molecule. Web lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Your solution’s ready to go! Lewis electron dot diagrams use dots to represent valence electrons around. Web draw the lewis dot structure of a given molecule or ion. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. С с c 119 х. Web lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Web to draw the lewis structure of an atom, write the symbol of. С с c 119 х. Draw the lewis dot diagram for a h' cation. ⚛ lewis electron dot structures. Make sure charges are in the diagram. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. Draw resonance structures of some molecules. Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Web ⚛ lewis dot diagrams. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. Valence electrons occupy the highest energy level (also known as the valence shell). Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. Web lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. So let's say we wanted to draw the dot structure for this molecule, so silicon tetrafluoride. Web draw the lewis dot structure of a given molecule or ion. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. Web lewis dot structures can be drawn to show the valence electrons that surround an atom itself. Draw the lewis dot diagram for a h' cation. A lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons:
H Lewis Dot Structure
How to Draw Lewis Dot Structure

rules for drawing lewis dot structures for molecules

Complete And Correctly Sequence The Steps For Drawing Lewis Structures

Lewis Dot Structure Definition, Examples, and Drawing

3 Ways to Draw Lewis Dot Structures wikiHow
Draw The Lewis Dot Diagram For A H+ Cation Alexander Blog
SOLVED Draw the Lewis dot diagram for a H^+cation.

Lewis Dot Structures of Atoms and Ions YouTube

Lewis Dot Structure Definition, Examples, and Drawing
Lewis Electron Dot Diagrams Use Dots To Represent Valence Electrons Around An Atomic Symbol.
See The Following Examples For How To Draw Lewis Dot Structures For Common Atoms Involved In Covalent Bonding.
Figure \(\Pageindex{1}\) Shows The Lewis Symbols For The Elements Of The Third Period Of The Periodic Table.
Web Draw The Lewis Dot Diagram For A H+ Cation.
Related Post: