Draw The Electron Configuration For A Neutral Atom Of Zinc, We add electrons to fill the outermost orbital that is occupied, and then add.
Draw The Electron Configuration For A Neutral Atom Of Zinc - The shorthand electron configuration (or noble gas configuration) as well as full electron. Web atomic number of zn = 30. We have chosen to show the full, unabbreviated configurations to provide more practice for students who want it,. The atomic number of zinc is 30, which means that all zinc atoms have 30 protons in their nuclei. We first need to find the number of electrons for the zn atom. Determine the electron configuration of ions. The atomic number of zinc is 30, which means that the neutral atom has equal numbers of protons and electrons, so a neutral atom of zinc would have 30 electrons. Web electron configuration chart of all elements is mentioned in the table below. Web to write the configuration for the zinc and the zinc ion, first we need to write the electron configuration for just zinc (zn). This indicates that zinc has the same electronic structure as the noble gas. For example, the electron configuration of lithium, 1 s ²2 s ¹, tells us that lithium. The shorthand electron configuration (or noble gas configuration) as well as full electron. Web this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Web for hydrogen, therefore, the single electron is placed. Justify the anomalies of the electron configurations in transition metals using magnetism experimental data. Web the electronic configuration of anions is assigned by adding electrons according to aufbau principle. Web electron configuration chart of all elements is mentioned in the table below. #_30^65zn# the zinc atom has 30 protons #=># 30 electrons. For example, the electron configuration of lithium, 1. We have chosen to show the full, unabbreviated configurations to provide more practice for students who want it,. The electron configuration for a neutral atom of zinc ( zn) can be written by. Web to write the configuration for the zinc and the zinc ion, first we need to write the electron configuration for just zinc (zn). Justify the anomalies. Web atomic number of zn = 30. Determine the electron configuration of ions. For example, the electron configuration of lithium, 1 s ²2 s ¹, tells us that lithium. Web this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. View the full answer step 2. Web first, write out the electron configuration for each parent atom. Web the atom is electrically neutral. The electron configuration for a neutral atom of zinc ( zn) can be written by. The atomic number of zinc is 30, which means that all zinc atoms have 30 protons in their nuclei. #_30^65zn# the zinc atom has 30 protons #=># 30. Web the electron configuration of zn2+ is 1s22s22p63s23p63d10. Web what are electron configurations? For example, the electron configuration of lithium, 1 s ²2 s ¹, tells us that lithium. This indicates that zinc has the same electronic structure as the noble gas. Identify and explain exceptions to predicted electron configurations for atoms and ions. Justify the anomalies of the electron configurations in transition metals using magnetism experimental data. The electron configuration for a neutral atom of zinc ( zn) can be written by. Typically, you need at least 8 steps to. Web to understand this concept, it's useful to write an example configuration. Predict the charge of common metallic and nonmetallic elements, and write. Web the electron configuration of zn2+ is 1s22s22p63s23p63d10. Web by “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. We add electrons to fill the outermost orbital that is occupied, and then add. Web atomic number of zn = 30. Electron configurations are an organized means of documenting. Web what are electron configurations? Justify the observed charge of ions to their electronic configuration. Typically, you need at least 8 steps to. Web this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Electron configurations are an organized means of documenting the placement of electrons based upon the. The atomic number of zinc is 30, which means that all zinc atoms have 30 protons in their nuclei. For example, the electron configuration of lithium, 1 s ²2 s ¹, tells us that lithium. The electron configuration for a neutral atom of zinc ( zn) can be written by. This indicates that zinc has the same electronic structure as. Web electron configurations describe where electrons are located around the nucleus of an atom. Web by “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. Web the electronic configuration of anions is assigned by adding electrons according to aufbau principle. Electron configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals. Predict the charge of common metallic and nonmetallic elements, and write their electron configurations. #_30^65zn# the zinc atom has 30 protons #=># 30 electrons. Justify the anomalies of the electron configurations in transition metals using magnetism experimental data. We first need to find the number of electrons for the zn atom. Web the electron configuration of zn2+ is 1s22s22p63s23p63d10. Typically, you need at least 8 steps to. We add electrons to fill the outermost orbital that is occupied, and then add. Web for hydrogen, therefore, the single electron is placed in the 1s orbital, which is the orbital lowest in energy (figure \(\pageindex{1}\)), and the electron configuration is written as. The electron configuration for a neutral atom of zinc (zn) can be written as [ar]4s²3d¹⁰. Web atomic number of zn = 30. Web first, write out the electron configuration for each parent atom. We have chosen to show the full, unabbreviated configurations to provide more practice for students who want it,.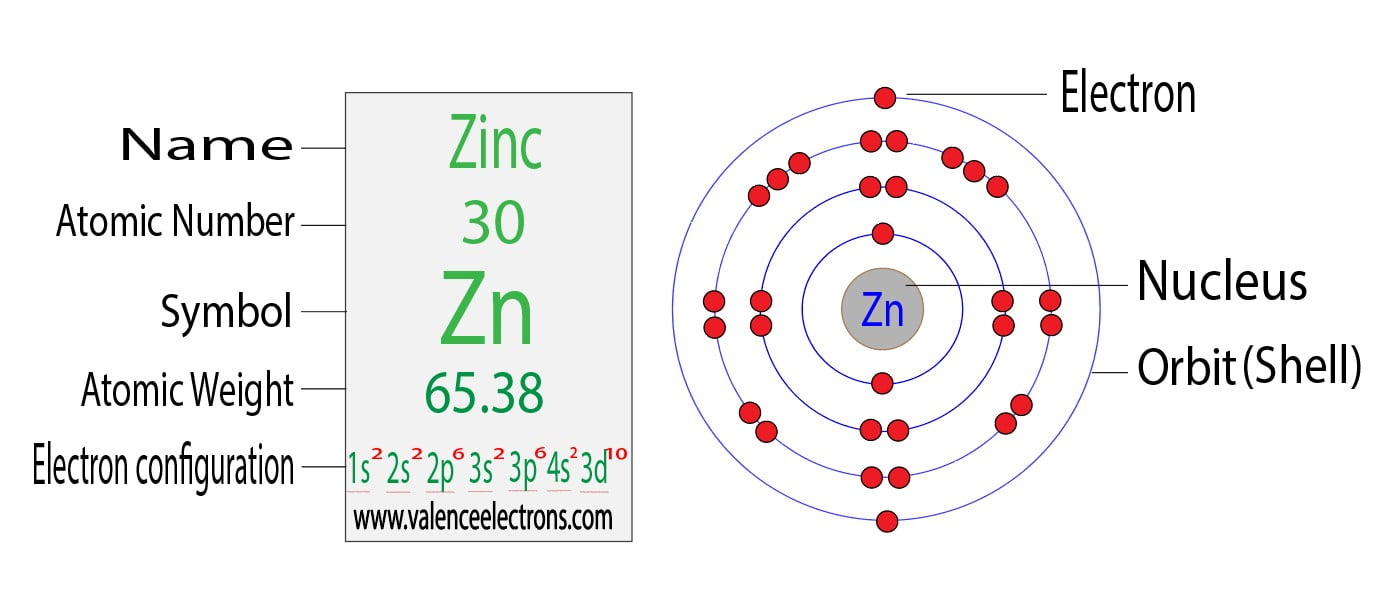
Gold(Au) electron configuration and orbital diagram

Draw The Electron Configuration For A Neutral Atom Of Zinc. Drawing
zinc electronic configuration How to Write Zinc electronic
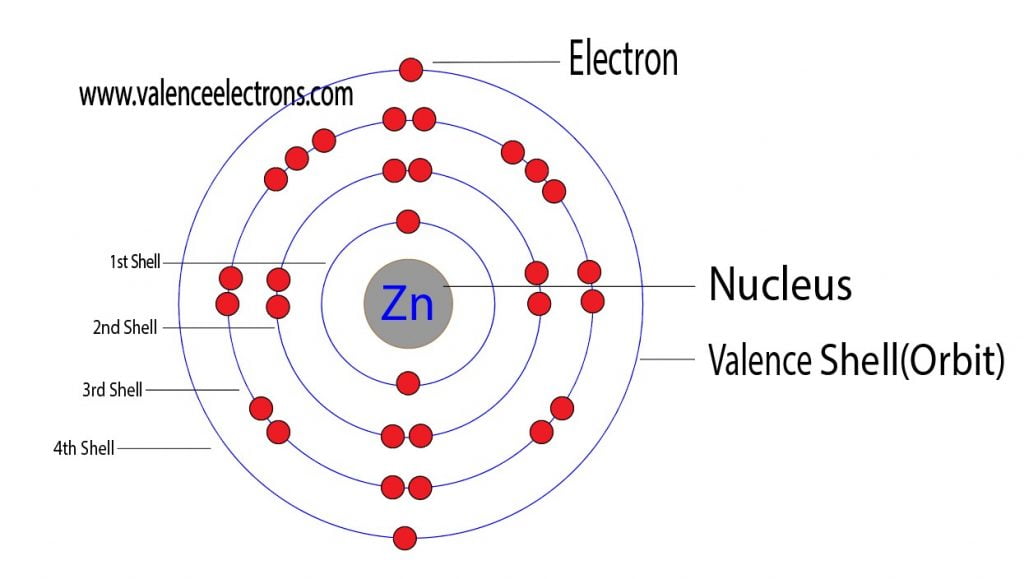
How Many Valence Electrons Does Zinc (Zn) Have?
Diagram Of Zinc Atom

Zinc electron configuration Stock Image C029/5029 Science Photo
:max_bytes(150000):strip_icc()/Zinc-58b6020f3df78cdcd83d332a.jpg)
Atom Diagrams Electron Configurations of the Elements
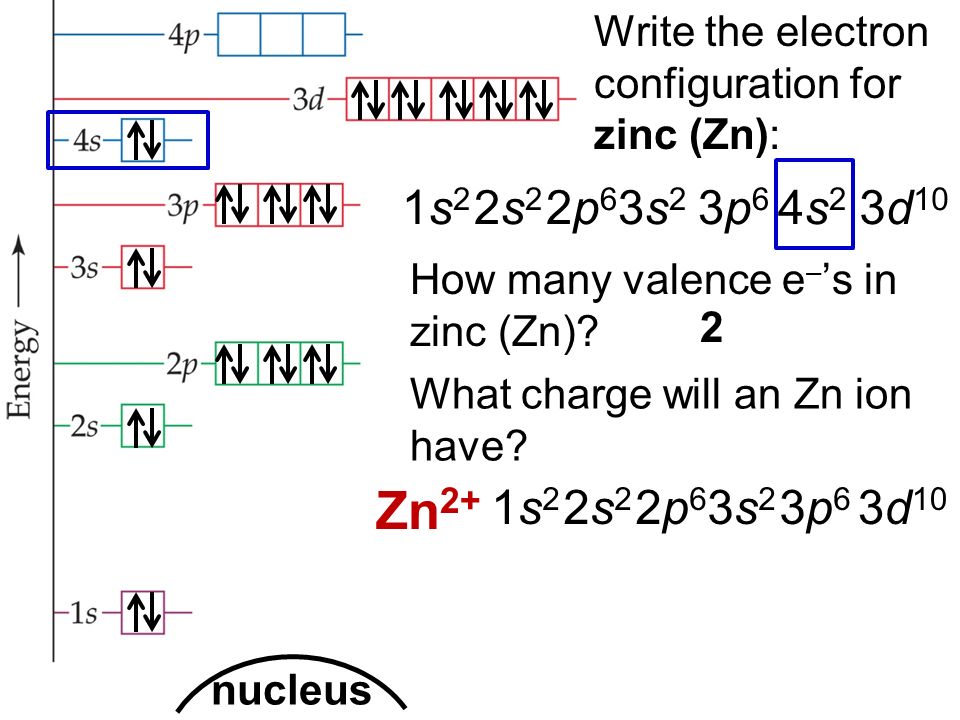
How To Find A Electron Configuration For Zinc Dynamic Periodic Table
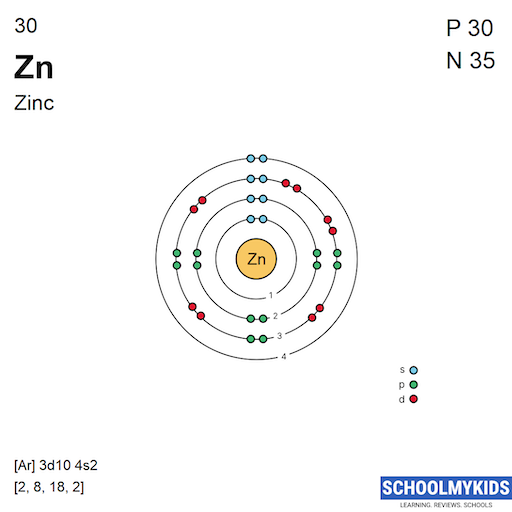
Zinc Electron Configuration

Draw The Electron Configuration For A Neutral Atom Of Zinc. Drawing
Web Electron Configuration Chart Of All Elements Is Mentioned In The Table Below.
The Atomic Number Of Zinc Is 30, Which Means That All Zinc Atoms Have 30 Protons In Their Nuclei.
Web What Are Electron Configurations?
The Electron Configuration For A Neutral Atom Of Zinc ( Zn) Can Be Written By.
Related Post:
