Draw The Electron Configuration For A Neutral Atom Of Sulfur, Differentiate between (spdf) electron configuration, orbital box diagram, and nobel gas configuration.
Draw The Electron Configuration For A Neutral Atom Of Sulfur - Web the electron configuration for a neutral sulfur atom seems to suggest that it takes eight electrons to fill the 3s and 3p orbitals in the valence shell of this atom. Web a good starting point when looking for the electron configuration of an ion is the electron configuration of the neutral atom. Web to write an electron configuration for a cation, start by writing the electron configuration for the neutral atom. Web electron configuration chart of all elements is mentioned in the table below. Web in order to write the sulfur electron configuration we first need to know the number of electrons for the s atom (there are 16 electrons). Web the electron configuration of an element is the arrangement of its electrons in its atomic orbitals. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the table. Web determine the atomic number of sulfur to ascertain the number of electrons for a neutral atom. Electron configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. It denotes a full s orbital. By knowing the electron configuration of an element, we can predict and explain a great deal of its chemistry. Web the arrangement of electrons in sulfur in specific rules in different orbits and orbitals is called the electron configuration of sulfur. (the loss of a single electron results in a +1 charge, the loss of two electrons results in a. Web electron configuration chart of all elements is mentioned in the table below. Otherwise, write the order of the energy levels with electron configuration chart: Identify the atomic number of sulfur to determine the total number of electrons for the neutral atom. Web to write the electron configuration of an atom, identify the energy level of interest and write the. An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Draw the electron configuration for a neutral atom of sulfur. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of electrons. The electron configuration for the first 10 elements. There are. Differentiate between (spdf) electron configuration, orbital box diagram, and nobel gas configuration. Web what are electron configurations? This configuration can be determined through various methods, including the aufbau principle, periodic table organization, bohr model representation, or orbital diagram visualization. Identify and explain exceptions to predicted electron configurations for atoms and ions. Web to write an electron configuration for a cation,. Then determine the number of electrons that were lost to form the cation. It is the fifth most common element by mass on earth and tenth in the universe. Sulfur chemically reacts with all elements except for platinum, gold, tellurium, iridium, and the noble gases. Web find the atomic number of nitrogen (7) and use this electron configuration calculator to. By knowing the electron configuration of an element, we can predict and explain a great deal of its chemistry. Web to write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: Orbital diagrams are pictorial representations of the electron configuration, showing the. It denotes a full s orbital. Web the electron configuration of an element is the arrangement of its electrons in its atomic orbitals. Web find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned. Web sulfur electron configuration. 7k views 3 years ago general chemistry 1. Predict the charge of common metallic and nonmetallic elements, and write their electron configurations. The electron configuration of sulfur is 3s 2 3p 4, if the electron arrangement is through orbitals. Web the electron configuration for a neutral atom of sulfur is 1s² 2s² 2p⁶ 3s² 3p⁴. Identify and explain exceptions to predicted electron configurations for atoms and ions. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of electrons. Let me tell you how this interactive periodic table will help you in your studies. This is the electron configuration of helium; Web determine the atomic number of sulfur. Identify and explain exceptions to predicted electron configurations for atoms and ions. Sulfur chemically reacts with all elements except for platinum, gold, tellurium, iridium, and the noble gases. Elemental sulfur is a yellow bright crystalline solid at room temperature. Identify the atomic number of sulfur to determine the total number of electrons for the neutral atom. Web the electron configuration. Predict the charge of common metallic and nonmetallic elements, and write their electron configurations. Wichita state university | answered on 10/24/2023. Elemental sulfur is a yellow bright crystalline solid at room temperature. Web the electron configuration for a neutral atom of sulfur is 1s² 2s² 2p⁶ 3s² 3p⁴. Web the arrangement of electrons in sulfur in specific rules in different orbits and orbitals is called the electron configuration of sulfur. It denotes a full s orbital. Electron configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Otherwise, write the order of the energy levels with electron configuration chart: Web sulfur electron configuration. Interpreting the electron configuration of a neutral atom in noble gas notation. When we write the configuration we'll put all 16 electrons in orbitals around the nucleus of the sulfur atom. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of electrons. Web the electron configuration of an element is the arrangement of its electrons in its atomic orbitals. Web the sulfur electron configuration, represented as 3s 2 3p 4 or 1s 2 2s 2 2p 6 3s 2 3p 4, illustrates the arrangement of electrons within the atom. By knowing the electron configuration of an element, we can predict and explain a great deal of its chemistry.
Sulfur Atom Science Notes and Projects
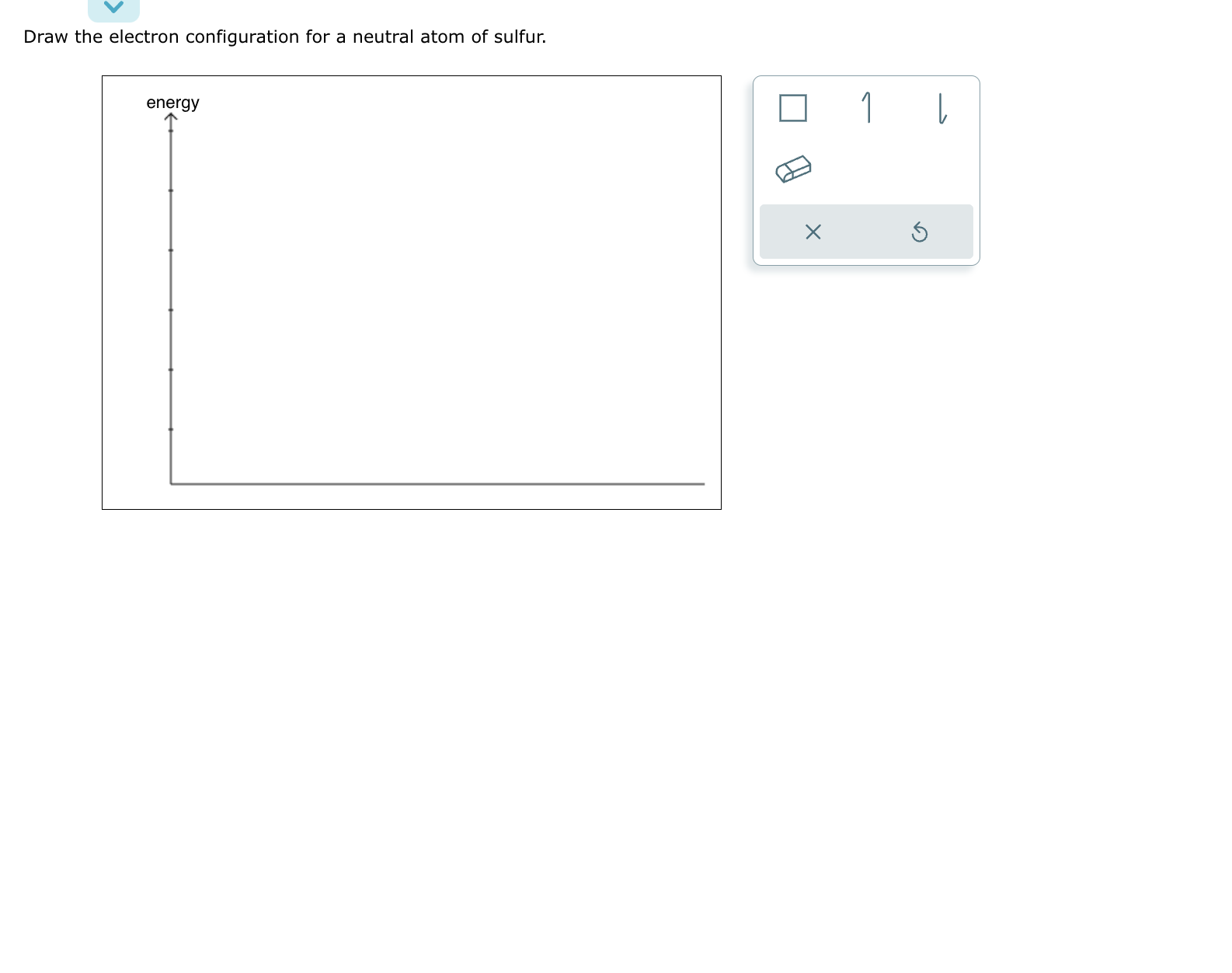
SOLVED Draw the electron configuration for a neutral atom of sulfur.

Sulfur Atom Diagram General Wiring Diagram
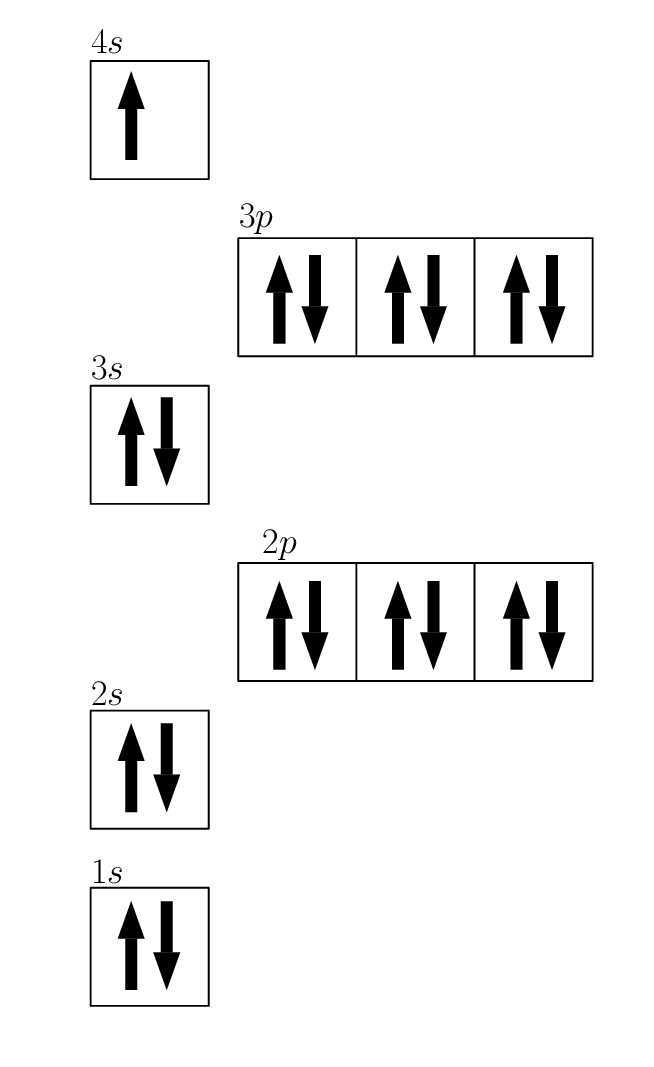
4.6 Electronic configuration The atom Siyavula

Sulfur S (Element 16) of Periodic Table Elements FlashCards
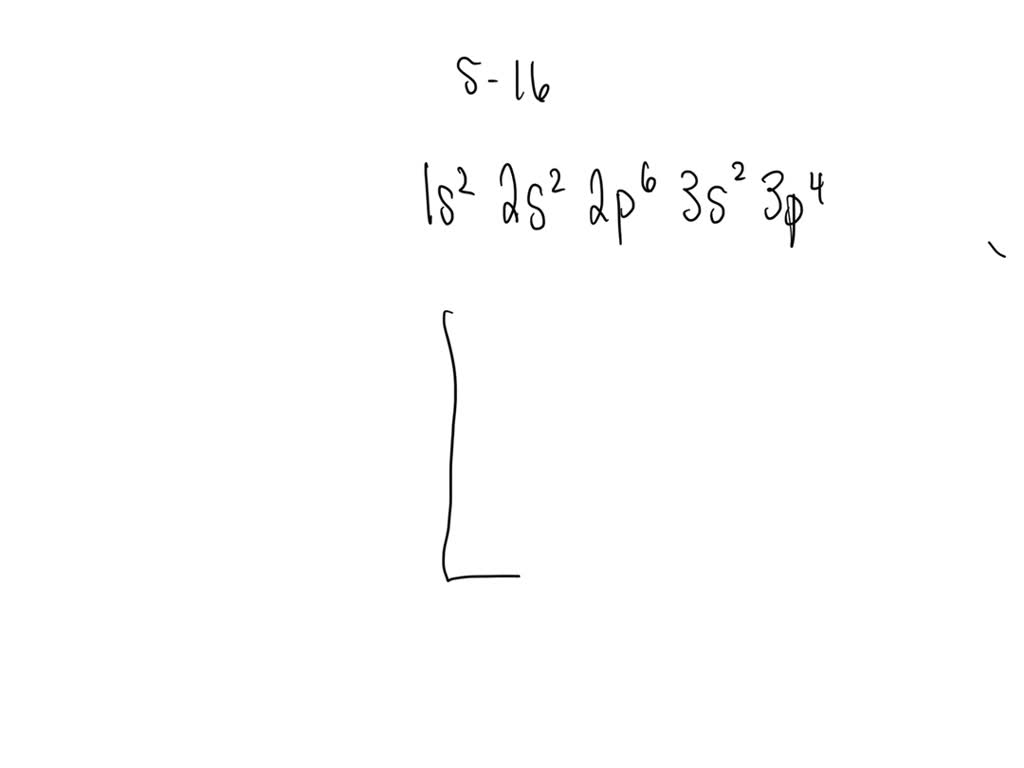
SOLVED Draw the electron configuration for a neutral atom of sulfur.
:max_bytes(150000):strip_icc()/sulfuratom-58b602563df78cdcd83d5a9d.jpg)
Atom Diagrams Electron Configurations of the Elements
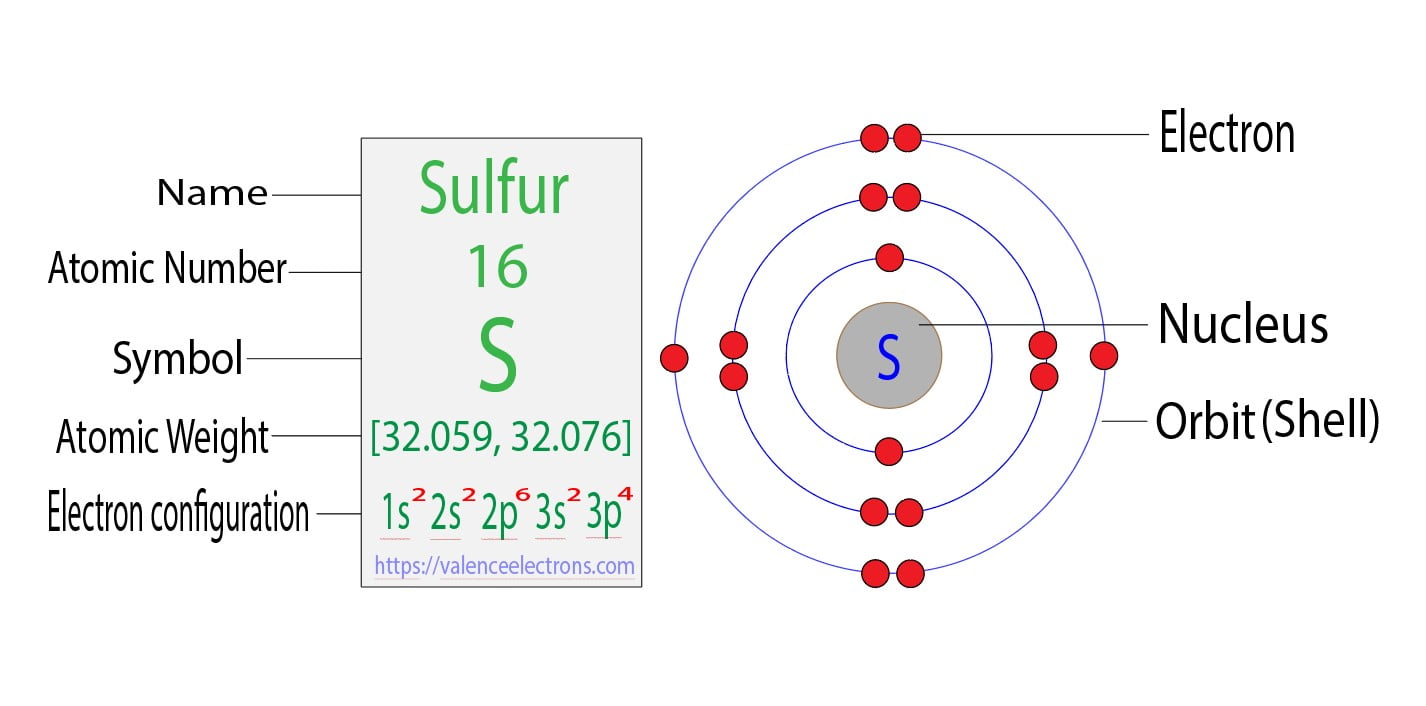
Electron Configuration Of Sulfur
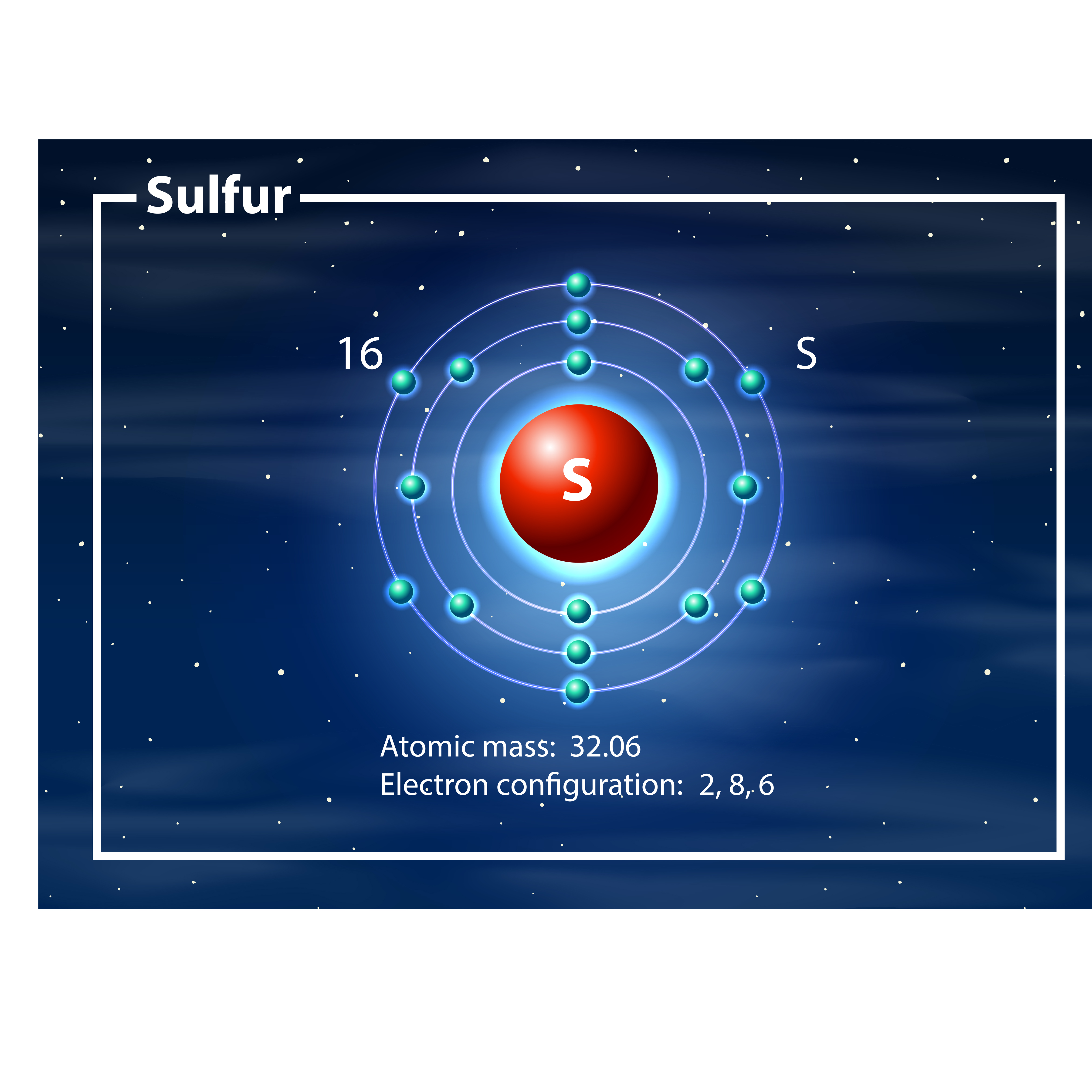
Electron Configuration Of Sulfur
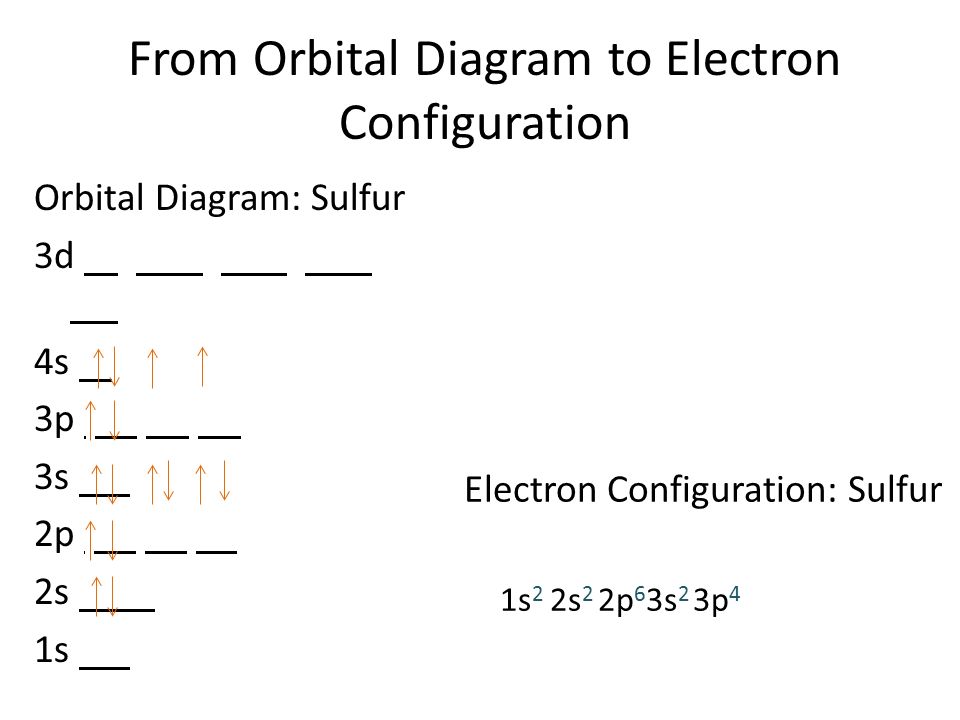
Sulfur Electron Configuration (S) with Orbital Diagram
Using The Formula For Electron Configuration, We Can Determine The Number Of Electrons That Can Occupy Each Energy Level.
Web Electron Configuration Chart Of All Elements Is Mentioned In The Table Below.
Web The Electron Configuration Of An Element Is The Arrangement Of Its Electrons In Its Atomic Orbitals.
Web Draw The Electron Configuration For A Neutral Atom Of Sulfur.
Related Post: