Draw The Electron Configuration For A Neutral Atom Of Lithium, Identify and explain exceptions to predicted electron configurations for atoms and ions.
Draw The Electron Configuration For A Neutral Atom Of Lithium - Web study with quizlet and memorize flashcards containing terms like what is the electron configuration of the neutral lithium atom?, what is the electron configuration. Because lithium’s final electron goes into the 2 s subshell, we write the. Web electron configurations describe where electrons are located around the nucleus of an atom. This makes it easier to. We know that the 1 s orbital can hold two of the electrons with their spins paired; Since 1s can hold only two electrons, the rest of the electron will enter the 2s orbital. Web the 2 s subshell holds a maximum of 2 electrons, and the 2 p subshell holds a maximum of 6 electrons. Because lithium’s final electron goes into the 2 s subshell, we. Web the next element is lithium, with z = 3 and three electrons in the neutral atom. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Web lithium has 3 electrons total.2 in the first (inner) shell1 in the second (outer, valence) shell.so the dot diagram has the symbol li with a single dot aroun. The electron configurations of elements with higher. Web lithium has an atomic number of 3, which means it has 3 electrons in its neutral state. Web the configuration notation provides an. The electron configuration for lithium is as follows: Web lithium has an atomic number of 3, which means it has 3 electrons in its neutral state. Web thus, the electron configuration and orbital diagram of lithium are: In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. An atom of the alkaline earth metal beryllium,. The shell diagram for a lithium atom (figure. Web the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Electrons are represented by dots or. Web electron configurations describe where electrons are located around the nucleus of an atom. Web this electron configuration calculator will instantly show. Since 1s can hold only two electrons, the rest of the electron will enter the 2s orbital. The electron configuration for lithium is as follows: For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Web the configuration notation provides an easy way for. An atom of the alkaline earth metal beryllium, with an atomic number of 4, contains four protons in the. The electron configuration for lithium is as follows: Table 5.2 shows the electron configurations of the elements with atomic numbers 1 through 18. 1s 2 2s 2 2p 1. Draw a lewis electron dot diagram for an atom or a monatomic. Because lithium’s final electron goes into the 2 s subshell, we write the. Then, add or remove electrons depending on the ion's charge. This makes it easier to. Web electron configurations describe where electrons are located around the nucleus of an atom. Electrons are represented by dots or. Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. The shell diagram for a lithium atom (figure. Web study with quizlet and memorize flashcards containing terms like what is the electron configuration of the neutral lithium atom?, what is the electron configuration. Lithium has an atomic number. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Electrons are represented by dots or. Web this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Because lithium’s final electron goes into. Write the electron configuration for a neutral atom of lithium. Web study with quizlet and memorize flashcards containing terms like what is the electron configuration of the neutral lithium atom?, what is the electron configuration. Then, add or remove electrons depending on the ion's charge. This makes it easier to. Table 5.2 shows the electron configurations of the elements with. Identify and explain exceptions to predicted electron configurations for atoms and ions. The first two electrons will fill the first energy level,. Write the electron configuration for a neutral atom of lithium. An atom of the alkaline earth metal beryllium, with an atomic number of 4, contains four protons in the. Web to find the electron configuration for an ion,. Web the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Web this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Web thus, the electron configuration and orbital diagram of lithium are: Web lithium has 3 electrons total.2 in the first (inner) shell1 in the second (outer, valence) shell.so the dot diagram has the symbol li with a single dot aroun. Lithium has an atomic number of 3, which means it has 3 electrons.step 2/42. Write the electron configuration for a neutral atom of lithium. Because lithium’s final electron goes into the 2 s subshell, we write the. Web study with quizlet and memorize flashcards containing terms like what is the electron configuration of the neutral lithium atom?, what is the electron configuration. The electron configurations of elements with higher. Then, add or remove electrons depending on the ion's charge. Electrons are represented by dots or. We know that the 1 s orbital can hold two of the electrons with their spins paired; The first two electrons will fill the first energy level,. Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Web the next element is lithium, with z = 3 and three electrons in the neutral atom.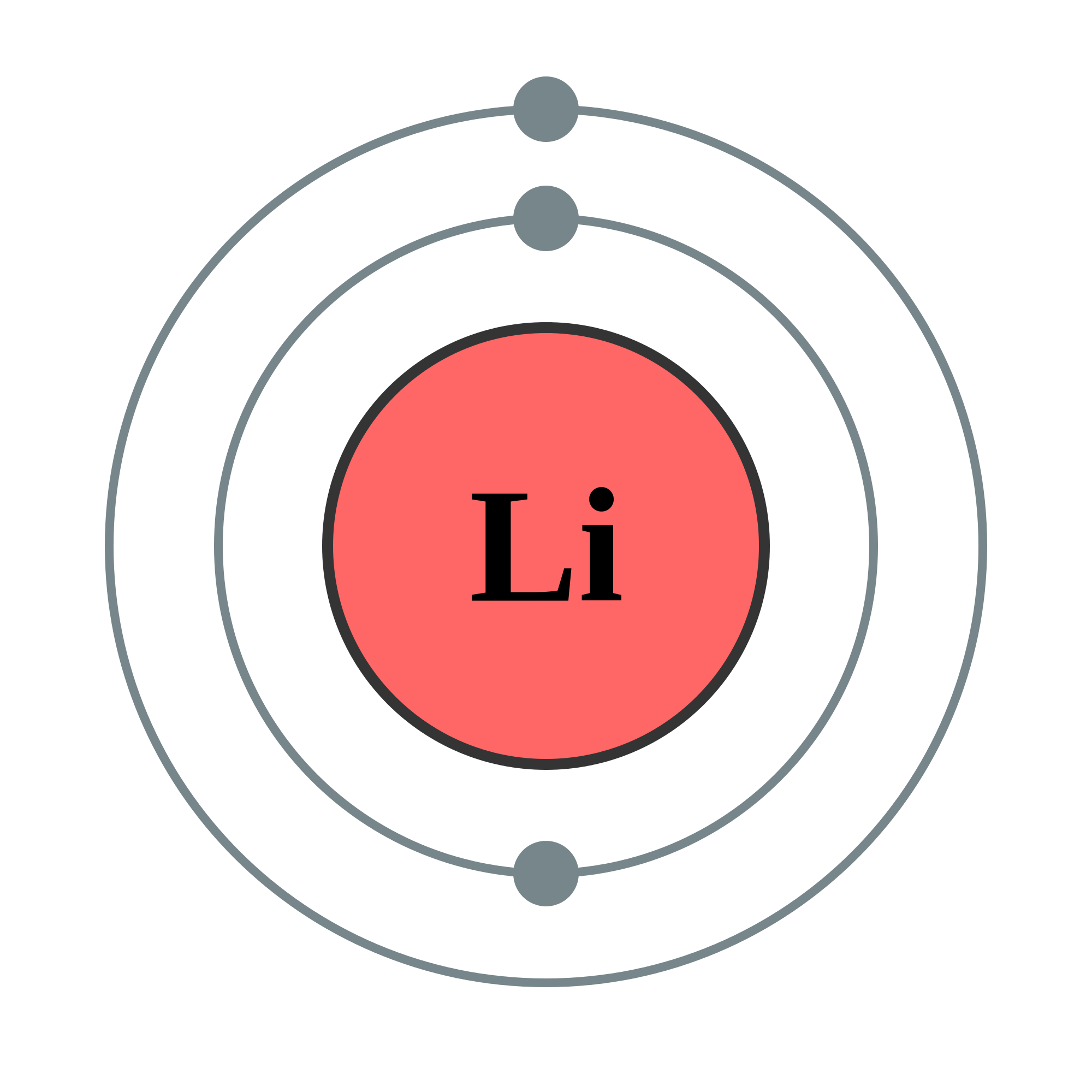
Lithium Elements Wiki
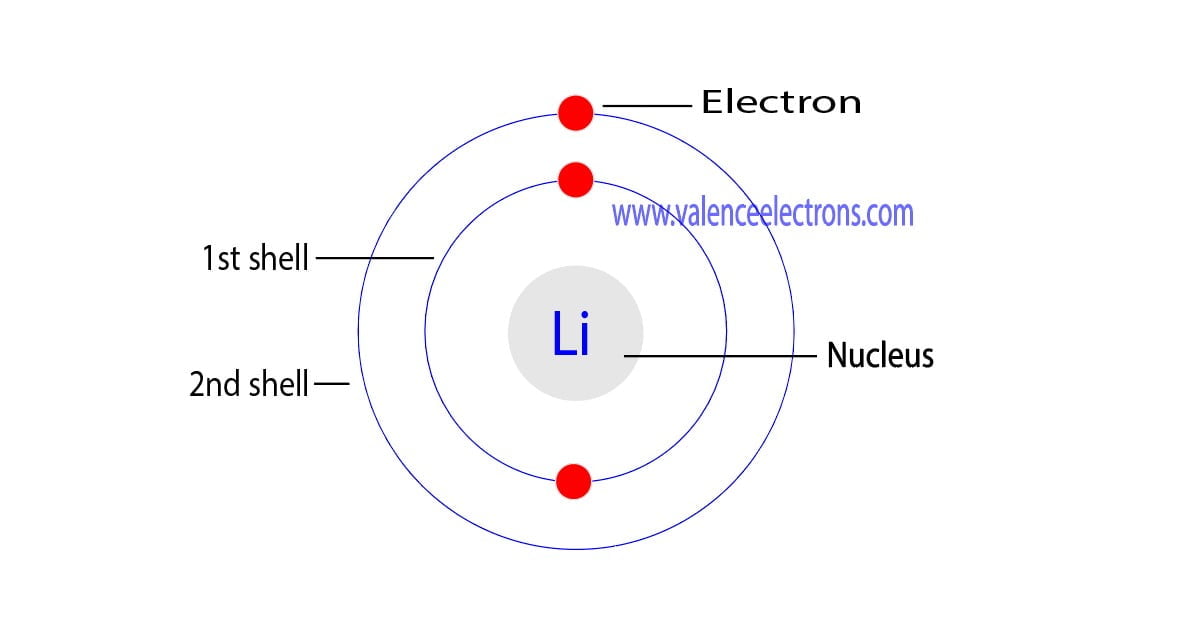
Lithium(Li) electron configuration and orbital diagram (2022)
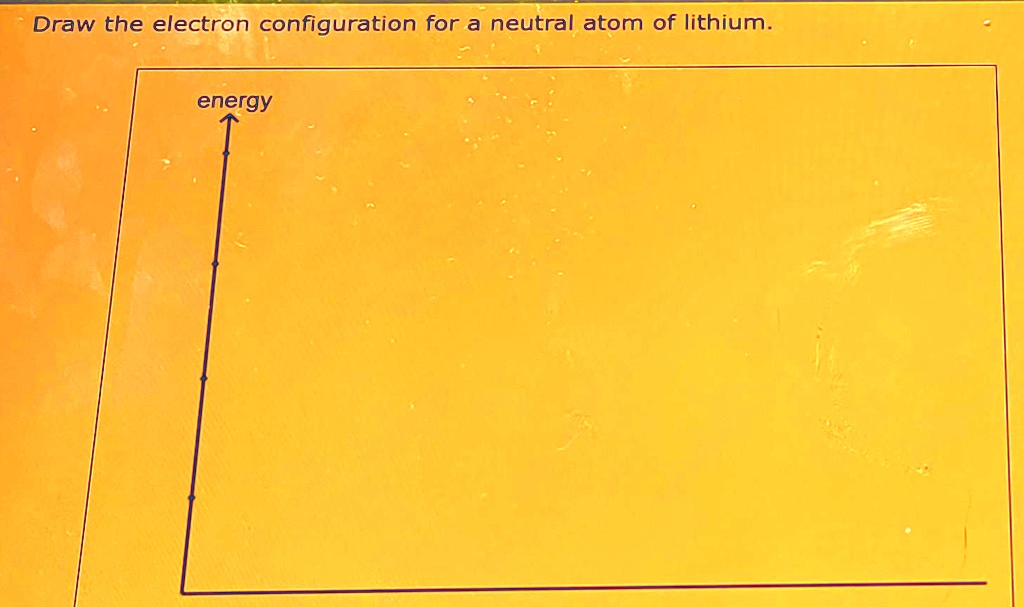
Draw the electron configuration for a neutral atom of lithium energy

Lithium electron configuration Stock Image C029/5021 Science
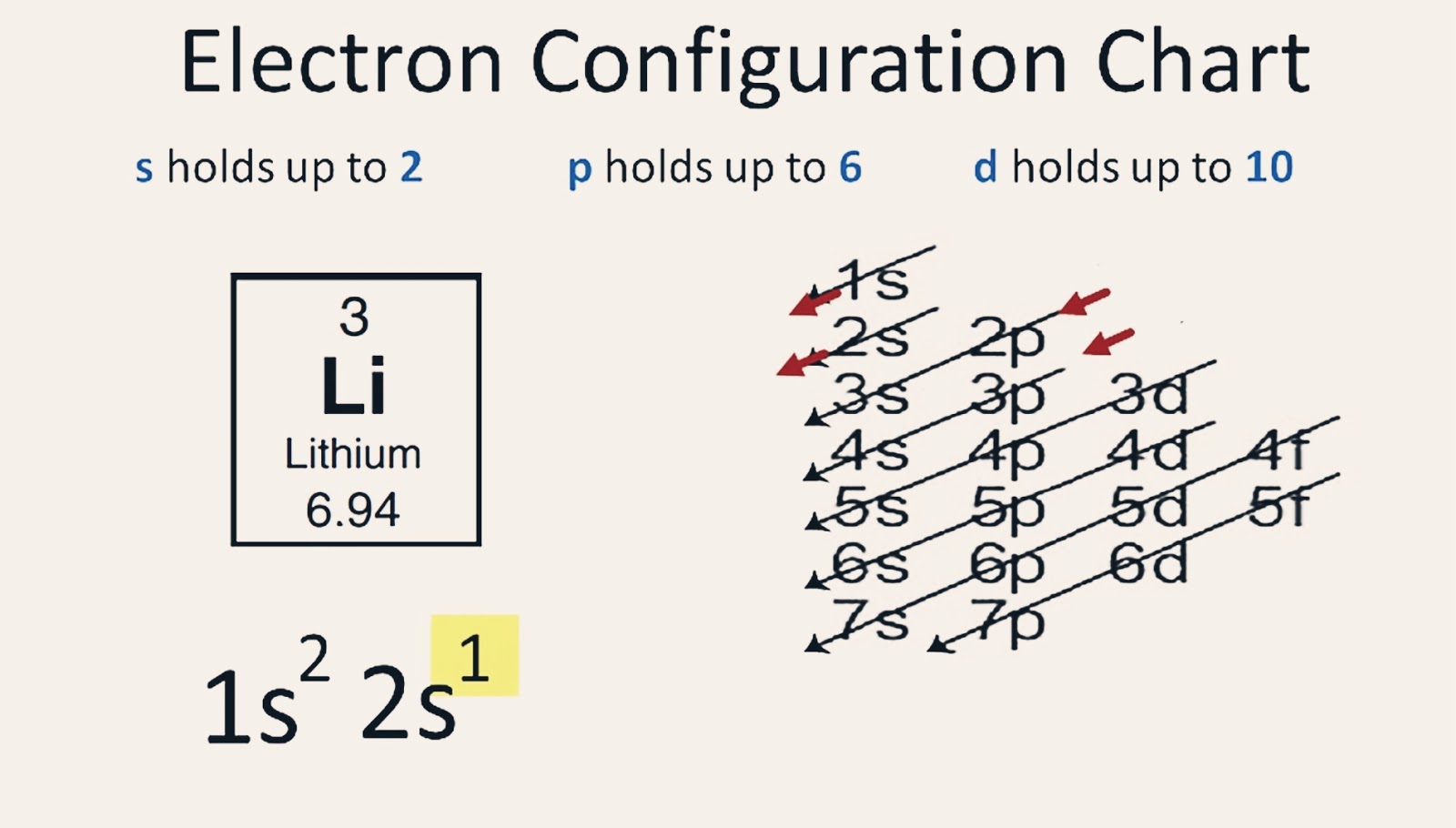
【5 Steps】Electron Configuration of Lithium(Li) in Just 5 Steps

Lithium atom Plugon
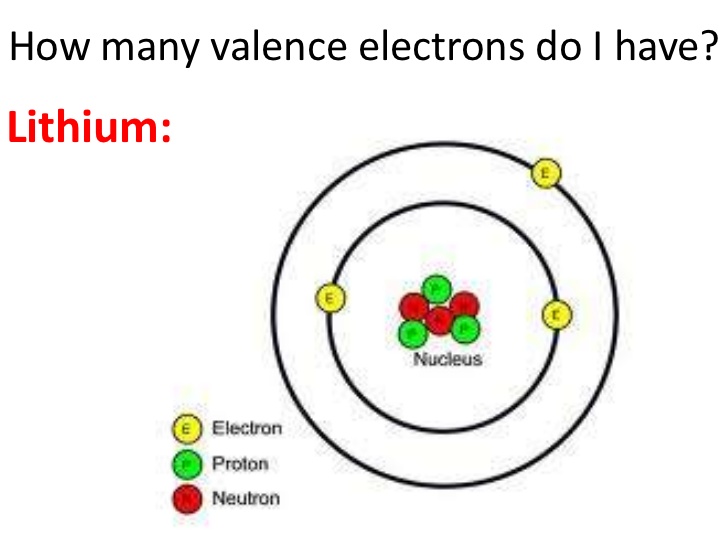
How Do We Can Find A Lithium Electron Configuration (Li)
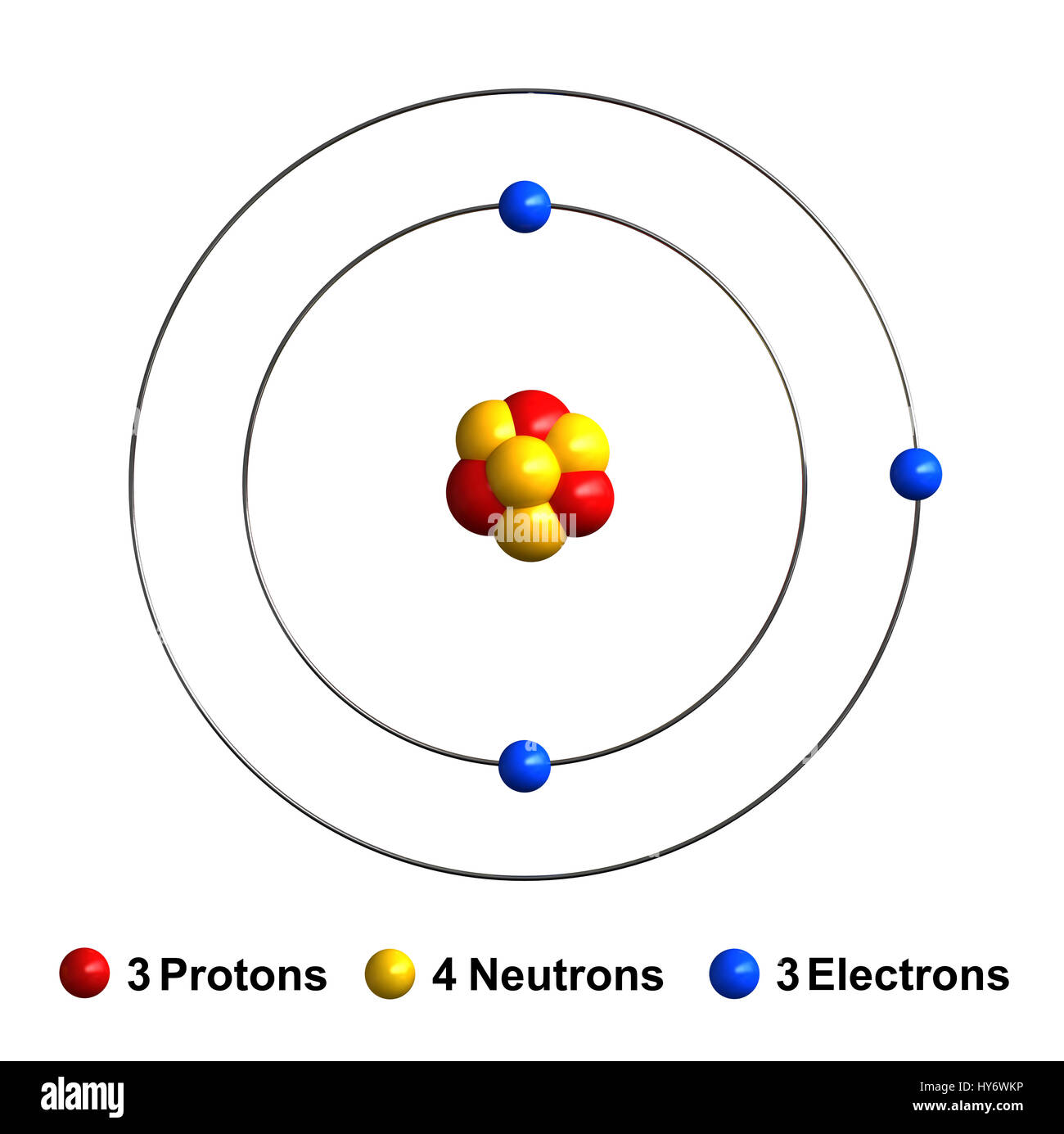
3d render of atom structure of lithium isolated over white background
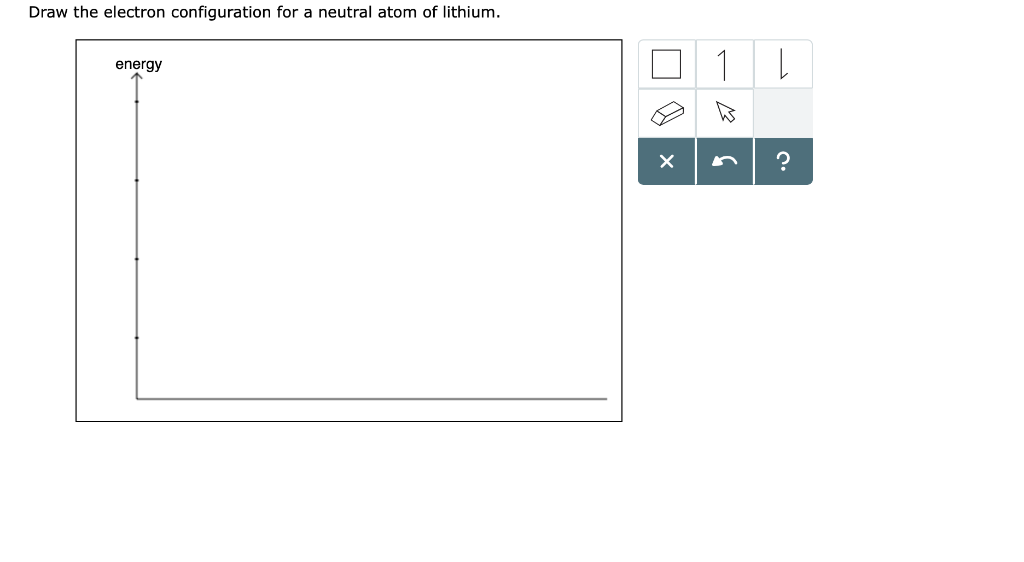
Solved Draw the electron configuration for a neutral atom of

Electron Dot Diagram For Neutral Lithium
Identify And Explain Exceptions To Predicted Electron Configurations For Atoms And Ions.
Web The 2 S Subshell Holds A Maximum Of 2 Electrons, And The 2 P Subshell Holds A Maximum Of 6 Electrons.
Web To Find The Electron Configuration For An Ion, First Identify The Configuration For The Neutral Atom.
Draw A Lewis Electron Dot Diagram For An Atom Or A Monatomic Ion.
Related Post: