Draw The Electron Configuration For A Neutral Atom Of Carbon, Web this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose.
Draw The Electron Configuration For A Neutral Atom Of Carbon - Web carbon has access to only #n = 2# and #n = 1#, so its six electrons can only go into the #1s#, #2s# and #2p# orbitals, from lowest to highest energy (aufbau. Web for example, take the electron configuration for carbon: Web this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. How do you write the condensed electron configurations for the following atoms, using the. The shorthand electron configuration (or noble gas configuration) as well as full. Identify and explain exceptions to predicted electron configurations for atoms and ions. Web electron configuration chart of all elements is mentioned in the table below. An electron configuration shows the distribution of electrons of an atom or a molecule. Nitrogen (atomic number 7) fills the 1 s and 2 s subshells and has one electron in each of the three 2 p orbitals, in. Web the electron configuration and orbital diagram for carbon are: Identify and explain exceptions to predicted electron configurations for atoms and ions. What is an electron configuration? Web by hund’s rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. Experimentally, it is found that the. Web carbon has access to only #n = 2#. Draw a lewis electron dot diagram for an atom or a monatomic ion. This makes it easier to. How do you write the condensed electron configurations for the following atoms, using the. Draw the orbital filling diagram for carbon and write its electron configuration. Typically, you need at least 8 steps to. Web electron configurations describe where electrons are located around the nucleus of an atom. Web for example, take the electron configuration for carbon: Web the electron configuration and orbital diagram for carbon are: Experimentally, it is found that the. This makes it easier to. Experimentally, it is found that the. Typically, you need at least 8 steps to. Web for example, take the electron configuration for carbon: Identify and explain exceptions to predicted electron configurations for atoms and ions. 2 electrons will pair up in the 1s orbital, 2 electrons pair up in the 2s orbital, and the remaining 2 electrons will be placed. In almost all cases, chemical bonds are formed by interactions of valence electrons in. This makes it easier to. Web electron configurations describe where electrons are located around the nucleus of an atom. An electron configuration shows the distribution of electrons of an atom or a molecule. Experimentally, it is found that the. Web by hund’s rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. Nitrogen (atomic number 7) fills the 1 s and 2 s subshells and has one electron in each of the three 2 p orbitals, in. Draw the orbital filling diagram for carbon. Experimentally, it is found that the. Web for example, take the electron configuration for carbon: Identify and explain exceptions to predicted electron configurations for atoms and ions. Web the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. How do you write the condensed electron configurations for. Web the electron configuration and orbital diagram for carbon are: The shorthand electron configuration (or noble gas configuration) as well as full. Experimentally, it is found that the. Draw a lewis electron dot diagram for an atom or a monatomic ion. What is an electron configuration? An electron configuration shows the distribution of electrons of an atom or a molecule. Draw a lewis electron dot diagram for an atom or a monatomic ion. What is an electron configuration? 2 electrons will pair up in the 1s orbital, 2 electrons pair up in the 2s orbital, and the remaining 2 electrons will be placed. Nitrogen (atomic number. 2 electrons will pair up in the 1s orbital, 2 electrons pair up in the 2s orbital, and the remaining 2 electrons will be placed. This makes it easier to. Nitrogen (atomic number 7) fills the 1 s and 2 s subshells and has one electron in each of the three 2 p orbitals, in. For example, the electron configuration. Experimentally, it is found that the. How do you write the condensed electron configurations for the following atoms, using the. Web by hund’s rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. Nitrogen (atomic number 7) fills the 1 s and 2 s subshells and has one electron in each of the three 2 p orbitals, in. In almost all cases, chemical bonds are formed by interactions of valence electrons in. Web the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Web carbon has access to only #n = 2# and #n = 1#, so its six electrons can only go into the #1s#, #2s# and #2p# orbitals, from lowest to highest energy (aufbau. Experimentally, it is found that the. Web electron configurations describe where electrons are located around the nucleus of an atom. What is an electron configuration? Web for example, take the electron configuration for carbon: Web the electron configuration for a neutral atom of carbon (c) is 1s² 2s² 2p². An electron configuration shows the distribution of electrons of an atom or a molecule. Web the electron configuration and orbital diagram for carbon are: Web electron configuration chart of all elements is mentioned in the table below. This makes it easier to.
Carbon Electron Configuration
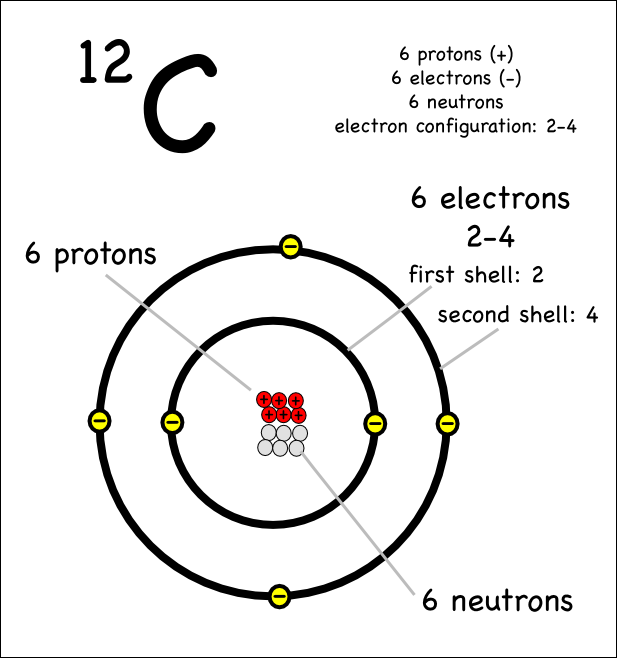
Drawing Atoms Montessori Muddle
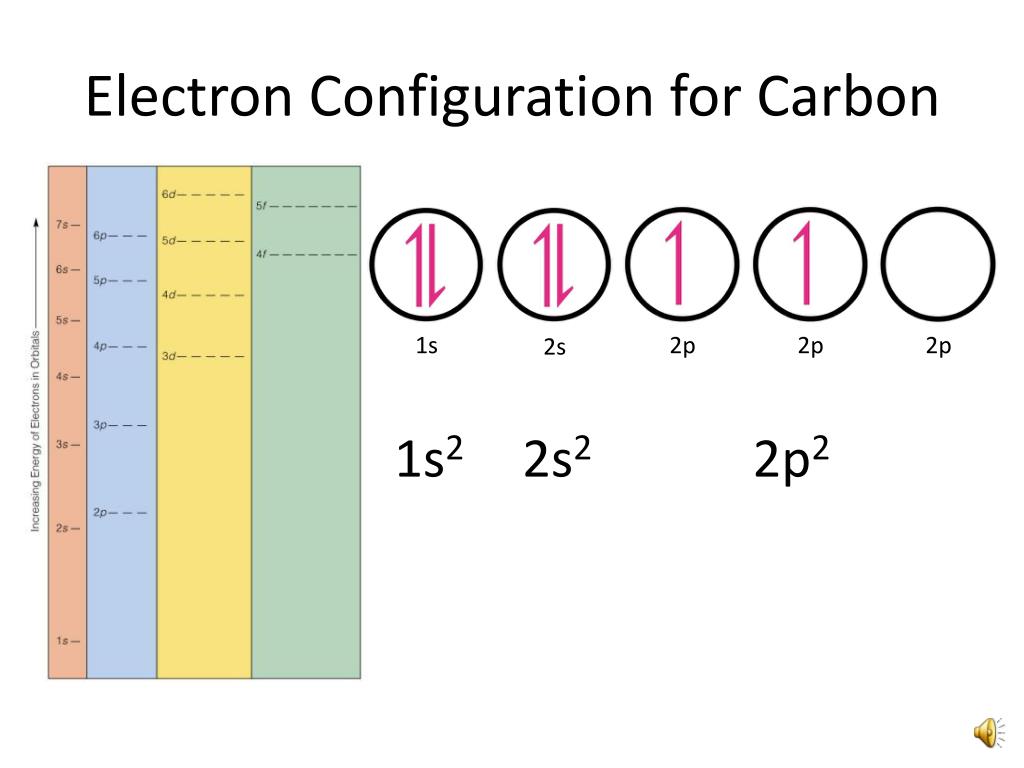
PPT Orbital Filling Electron Configurations PowerPoint Presentation

8.2i Writing the electron configuration of a neutral atom with s and p
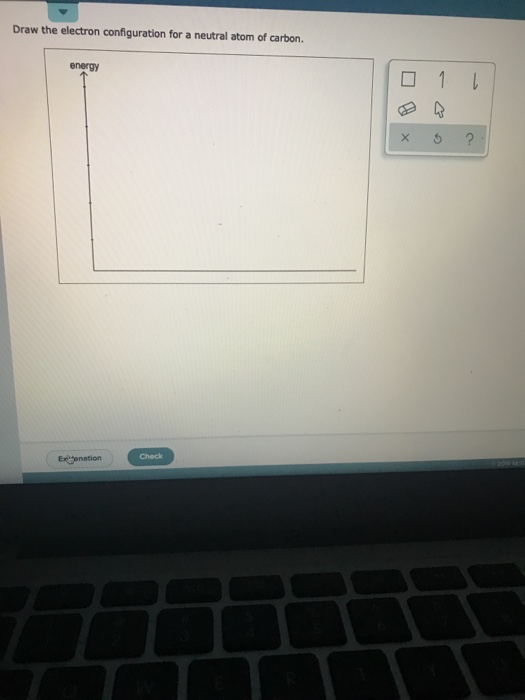
Solved Draw the electron configuration for a neutral atom of
Solved Draw the electron configuration for a neutral atom of

Orbital Diagram For Carbon (C) Carbon Electron Configuration
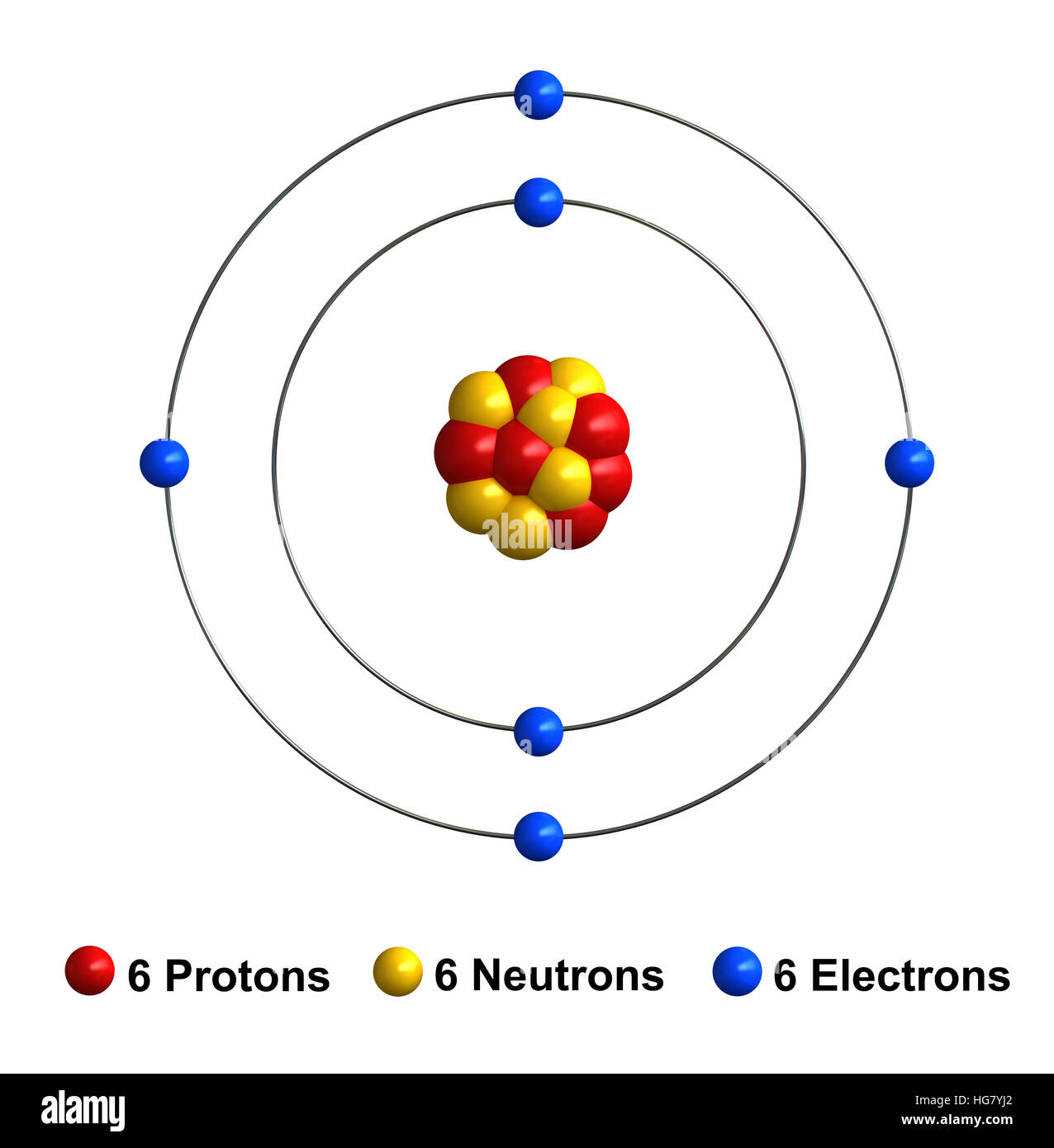
Labelled Diagram Of A Carbon Atom

Electron Configuration Chart

Carbon(C) electron configuration and orbital diagram
For Example, The Electron Configuration Of Lithium, 1 S ²2 S ¹, Tells Us That Lithium Has Two Electrons In The 1 S Subshell And One Electron In The 2 S Subshell.
In This Configuration, The Numbers Represent The Principal Energy Levels (Shells), And The Letters.
2 Electrons Will Pair Up In The 1S Orbital, 2 Electrons Pair Up In The 2S Orbital, And The Remaining 2 Electrons Will Be Placed.
Typically, You Need At Least 8 Steps To.
Related Post: