Draw The Electron Configuration For A Neutral Atom Of Beryllium, Let 4 be its atomic number (z=4).
Draw The Electron Configuration For A Neutral Atom Of Beryllium - Web this means that the electron configuration of beryllium responds to be 1s^2 2s^2. It describes how electrons are distributed among the various. We will discuss the electronic arrangement of beryllium with a detailed explanation of its ground state configuration, orbital diagram. 1 s 2 2 s 1 2 p 1. This makes it easier to. Web be2+ largely forms covalent compounds. 1 determine the atomic number of beryllium from the periodic table. Web the electron configuration for a neutral atom of beryllium is 1s² 2s² or [he] 2s². The first shell (also known as the 1s orbital) can hold up to. Web using s p d f notation, what is the electron configuration for a neutral atom of beryllium? Write the electron configuration for a neutral atom of beryllium. The first shell (also known as the 1s orbital) can hold up to. There are 2 steps to solve this one. To begin solving this, recall that the aufbau principle guides the process of adding electrons to an atom, filling up orbitals in an order of. First, identify the atomic. Figure 2.1.1), and the electron configuration is written as 1 s1 and. Web the electron configuration of beryllium refers to the arrangement of electrons in the beryllium atom’s orbitals. Electronegativity (pauling scale) the tendency of an atom to attract electrons. It describes how electrons are distributed among the various. Web the configuration notation provides an easy way for scientists to. Figure 2.1.1), and the electron configuration is written as 1 s1 and. 1 s 2 2 s 1 2 p 1. Web be2+ largely forms covalent compounds. Web electron configuration chart of all elements is mentioned in the table below. The first shell (also known as the 1s orbital) can hold up to. Web unless there is a reason to show the empty higher energy orbitals, these are often omitted in an orbital diagram: Web the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Identify and explain exceptions to predicted electron configurations for atoms and ions. Typically, you need. 1 s 2 2 s 1 2 p 1. It describes how electrons are distributed among the various. Solution for draw the electron configuration for a neutral atom. Web continuing on the periodic table to the next largest atom, beryllium, with 4 electrons, the electron configuration is 1s 2 2s 2. Now that the 2 s subshell is filled, electrons. There are 2 steps to solve this one. 1 s 2 2 s 2. Web the energy released when an electron is added to the neutral atom and a negative ion is formed. Web using s p d f notation, what is the electron configuration for a neutral atom of beryllium? The predominant oxidation state of beryllium is +2; 1 s 2 2 s 2. Web using s p d f notation, what is the electron configuration for a neutral atom of beryllium? Electronegativity (pauling scale) the tendency of an atom to attract electrons. Energy 1 l х 5 ? 1 s 2 2 s 2. Web the electron configuration of beryllium refers to the arrangement of electrons in the beryllium atom’s orbitals. Web a beryllium atom has the electronic configuration [he] 2s2. Web this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Beginning with the transition metal scandium (atomic. Web for a neutral. Web beryllium electron configuration using the aufbau principle. Write the electron configuration for a neutral atom of beryllium. 1 s 2 2 s 1 2 p 1. First, identify the atomic number of beryllium, which is 4, to. Web this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. First, identify the atomic number of beryllium, which is 4, to. Web be2+ largely forms covalent compounds. A beryllium atom is a neutral atom that has 4 atomic numbers which implies it has a total of 4 electrons. Web learn more about this topic, chemistry and related others by exploring similar questions and additional content below. Web the configuration notation. The predominant oxidation state of beryllium is +2; Electronegativity (pauling scale) the tendency of an atom to attract electrons. Web this means that the electron configuration of beryllium responds to be 1s^2 2s^2. Web this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Figure 2.1.1), and the electron configuration is written as 1 s1 and. 1 determine the atomic number of beryllium from the periodic table. Web a beryllium atom has the electronic configuration [he] 2s2. Let 4 be its atomic number (z=4). O electronic structure drawing a box diagram of the electron configuration of an atom draw the electron configuration for a. There are 2 steps to solve this one. This makes it easier to. First, identify the atomic number of beryllium, which is 4, to. In case the element is neutral, its atomic number will be similar. Web the energy released when an electron is added to the neutral atom and a negative ion is formed. We will discuss the electronic arrangement of beryllium with a detailed explanation of its ground state configuration, orbital diagram. Solution for draw the electron configuration for a neutral atom.
Beryllium(Be) electron configuration and orbital diagram
Solved Draw the electron configuration for a neutral atom of

Beryllium+Electron+Configuration_+Orbital+Diagram+Be_+1s22s2

FileElectron shell 004 Beryllium.svg Wikimedia Commons Atom

Be 2+ Electron Configuration (Beryllium Ion) YouTube
SOLVED Draw the electron configuration for a neutral atom of beryllium.
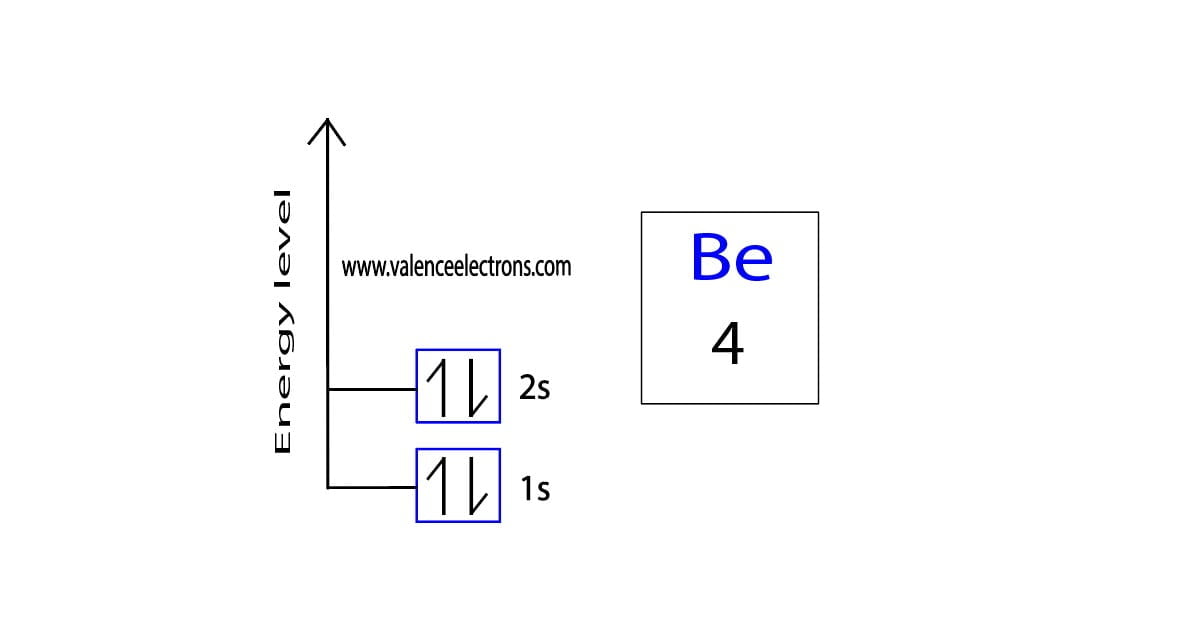
Orbital Diagram for Beryllium and Process of Drawing It
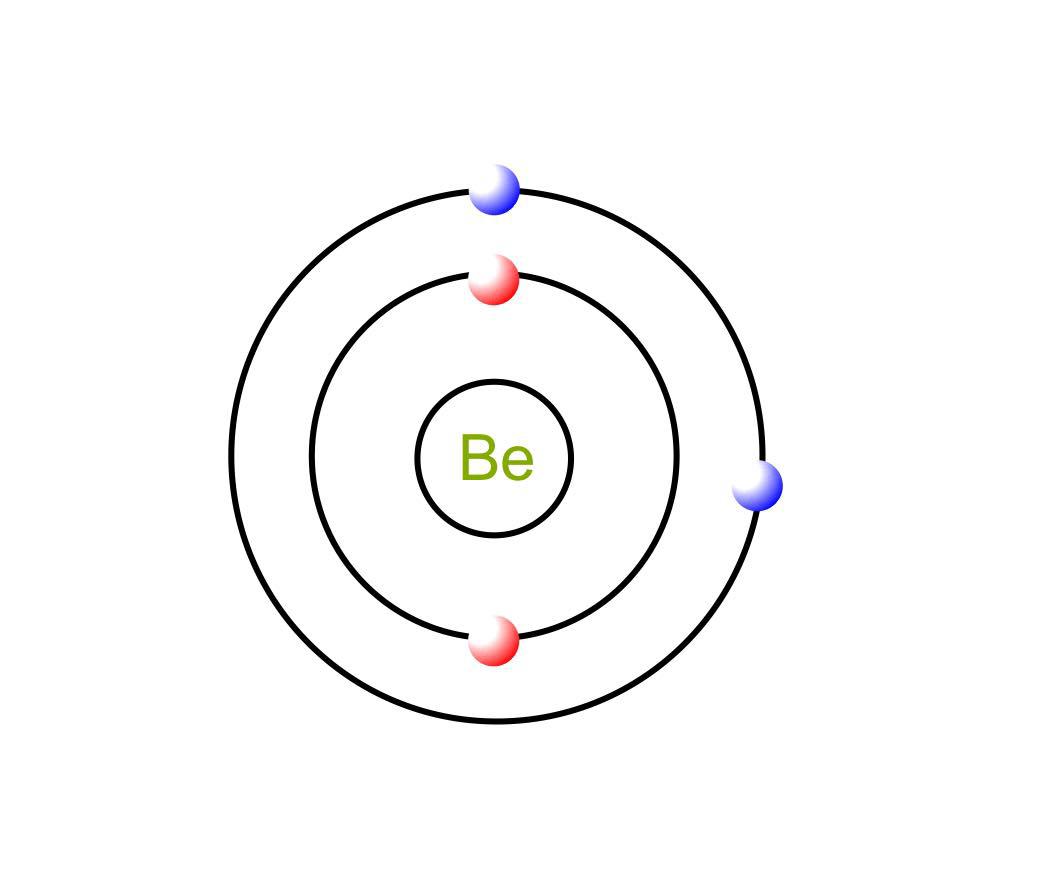
Draw a neutral Beryllium atom showing its electron shells. Quizlet

Beryllium Electron Configuration YouTube

Draw The Electron Configuration For A Neutral Atom Of Beryllium
Draw The Electron Configuration For A Neutral Atom Of Beryllium.
Web For A Neutral Atom Of Beryllium (Be), Which Has An Atomic Number Of 4, The Electron Configuration Is As Follows:
1 S 2 2 S 2.
1 S 2 2 S 1 2 P 1.
Related Post: