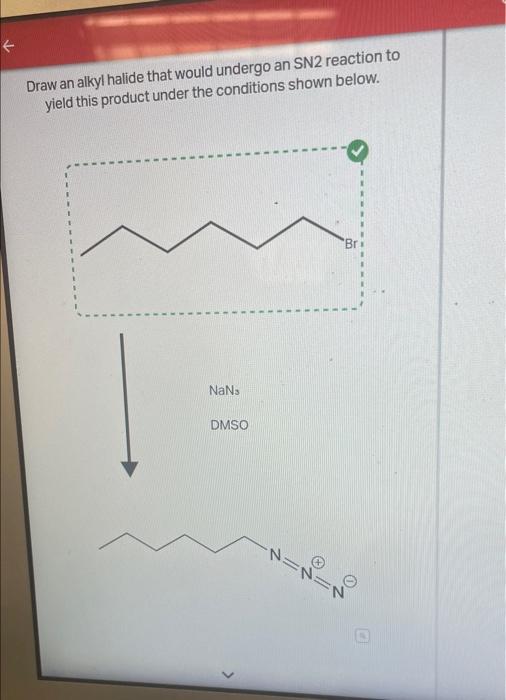
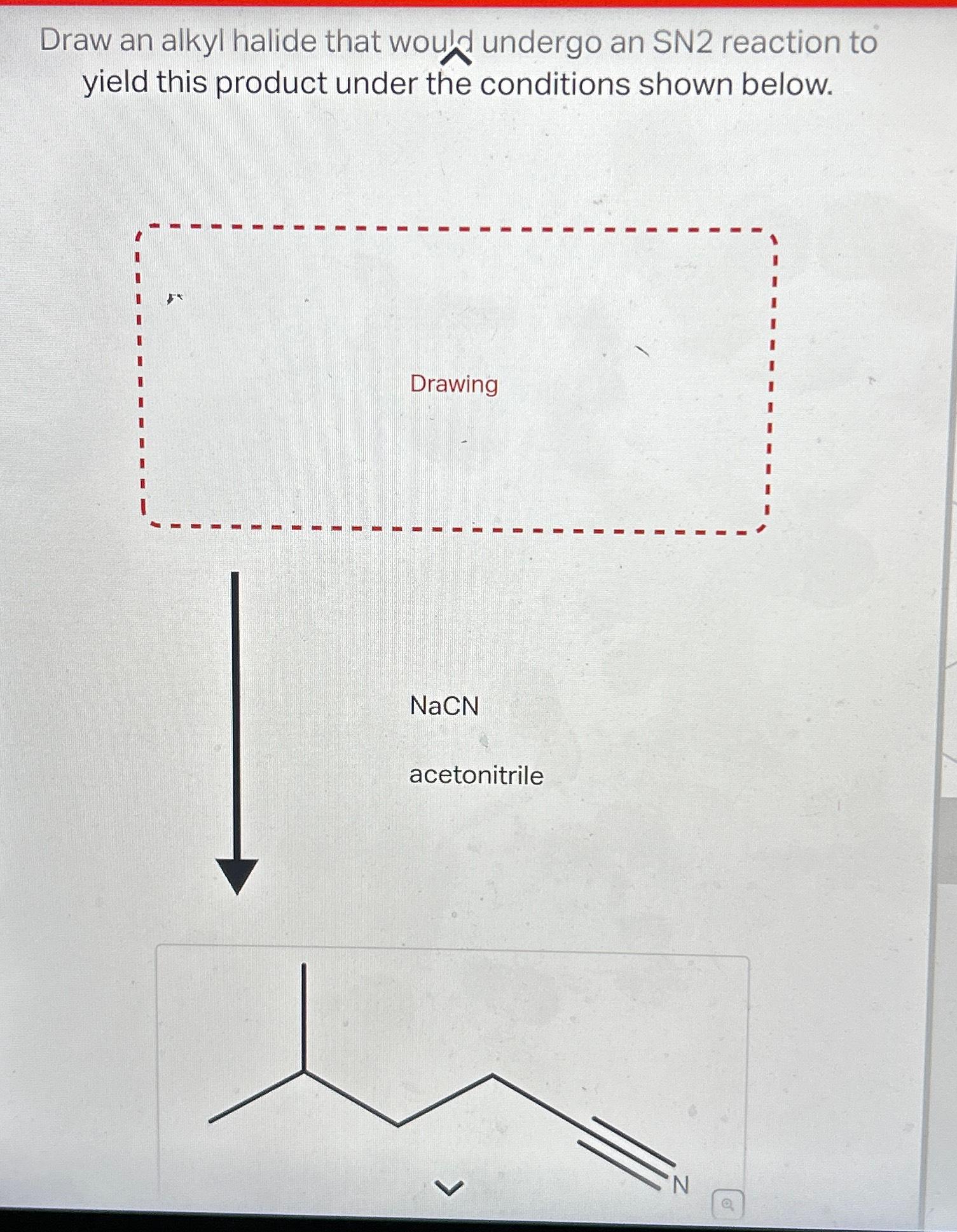
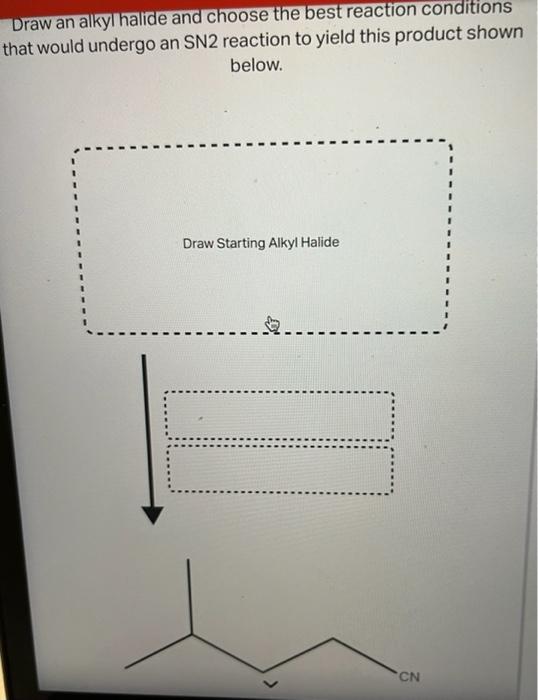
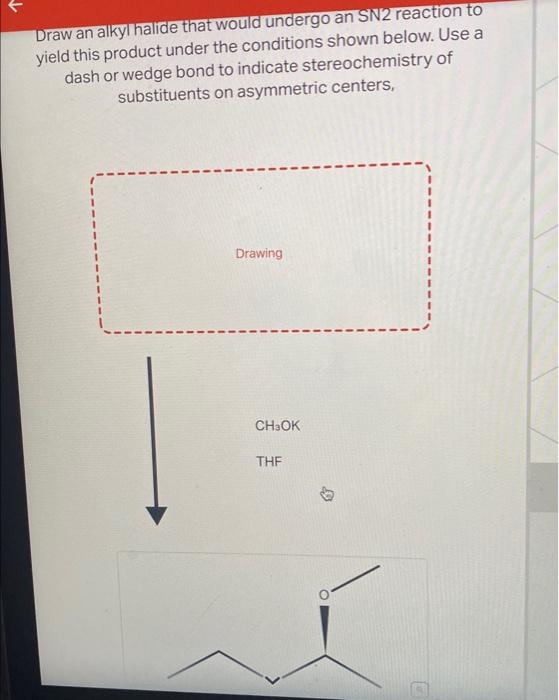
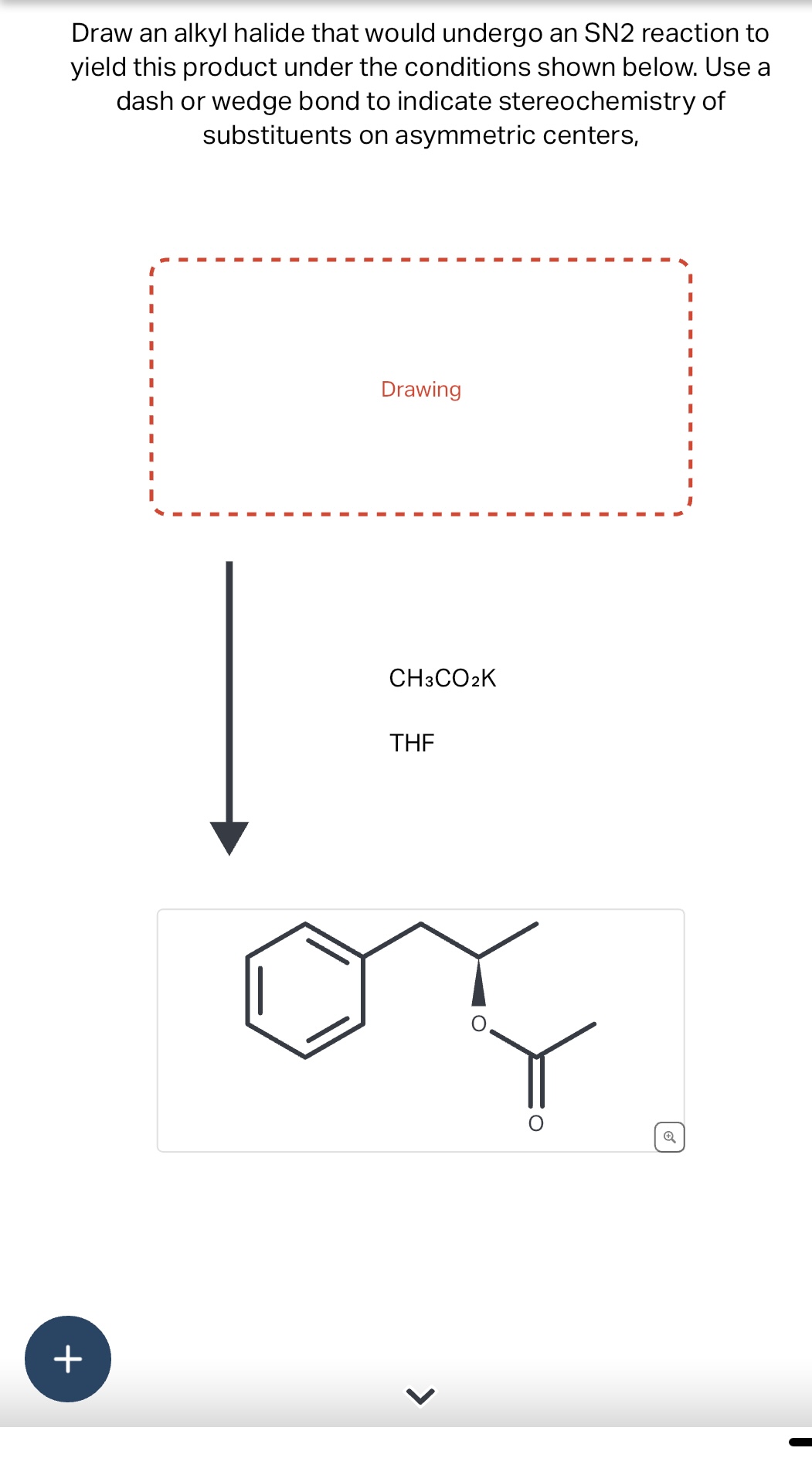
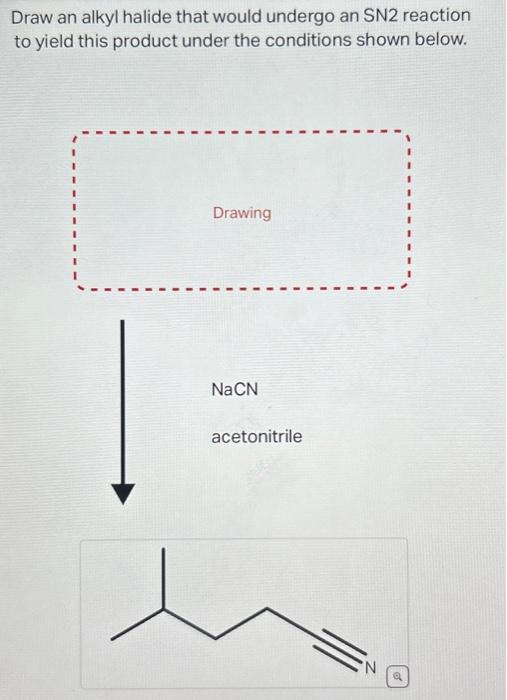
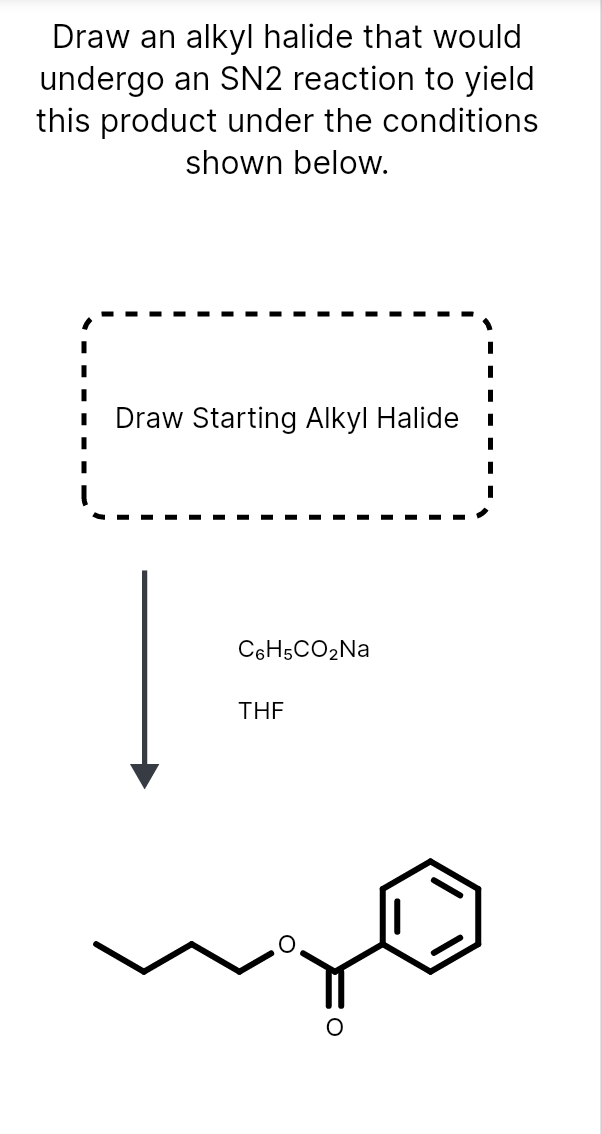
Draw An Alkyl Halide That Would Undergo An Sn2 Reaction, Web draw an alkyl halide that would undergo an sn2 reaction to yield this product under the conditions shown below.
Draw An Alkyl Halide That Would Undergo An Sn2 Reaction - Even though it is a primary alkyl halide, the rate of sn2 is way slower (about 10^5 times) compared to regular primary alkyl halides. Sn1 reactions depend on the stability of the cation formed when the leaving group had left. Web a stereospecific reaction is one in which different stereoisomers react to give different stereoisomers of the product. Suggest a reason why vinyl halides and aryl halides do. Draw an alkyl halide and choose the best reaction conditions that would undergo an. Tertiary > secondary > primary. Web draw an alkyl halide that would undergo an sn 2 reaction to. For the discussions on s n 2 mechanism so far, we focused on the reaction of methylbromide ch 3 br. Web discuss the role of steric effects in s n 2 reactions. Yield this product under the conditions shown below. Arrange a given series of alkyl halides in order of increasing or decreasing reactivity towards nucleophilic substitution through the s n 2 mechanism. Yield this product under the conditions shown below. Thf c h 3 c o 2 n a. Web turns out that the methyl halides and the primary alkyl halide react the fastest in an sn2 mechanism. Sn1. Bimolecular nucleophilic substitution (sn 2) reactions are concerted, meaning they are a one step process. Even though it is a primary alkyl halide, the rate of sn2 is way slower (about 10^5 times) compared to regular primary alkyl halides. Sn2 reactions depend on the fastness of the leaving group. 3) stability of the leaving group. Suggest a reason why vinyl. Web discuss the role of steric effects in s n 2 reactions. Use a dash or wedge bond to indicate the stereochemistry of substituents on asymmetric centers. Arrange a given series of alkyl halides in order of increasing or decreasing reactivity towards nucleophilic substitution through the s n 2 mechanism. For the discussions on s n 2 mechanism so far,. The nuclephile and electrophile must be correctly oriented for orbital overlap to occur and trigger chemical reactivity. Suggest a reason why vinyl halides and aryl halides do. Thf c h 3 c o 2 n a. 2) strength of the nucleophile. Web 1) structure of the alkyl halide. So, since tertiary carbocations are most stable of the three will undergo sn1 reaction easily. There are 2 steps to solve this one. Arrange a given series of alkyl halides in order of increasing or decreasing reactivity towards nucleophilic substitution through the s n 2 mechanism. As the nucleophile comes in on one side of the substrate and bonds to. Structure of the alkyl halide (substrate) and s n 2 reaction rates. There are 2 steps to solve this one. Yield this product under the conditions shown below. Bimolecular nucleophilic substitution (sn 2) reactions are concerted, meaning they are a one step process. So, since tertiary carbocations are most stable of the three will undergo sn1 reaction easily. As the nucleophile comes in on one side of the substrate and bonds to the. Web draw an alkyl halide that would undergo an sn2 reaction to yield this product under the conditions shown below. 3) stability of the leaving group. 2) strength of the nucleophile. Web the effect of alkyl halide structure on s n 2 reaction rate. Even though it is a primary alkyl halide, the rate of sn2 is way slower (about 10^5 times) compared to regular primary alkyl halides. Web draw an alkyl halide that would undergo an sn 2 reaction to. Web a stereospecific reaction is one in which different stereoisomers react to give different stereoisomers of the product. Use a dash or wedge. Web the sn2 mechanism proceeds through a concerted backside attack of a nucleophile upon an alkyl halide, and is fastest for methyl > primary > secondary> > 3° Draw an alkyl halide that would undergo an sn2 reaction to yield this product under the conditions shown below. Web the effect of alkyl halide structure on s n 2 reaction rate.. Tertiary > secondary > primary. Yield this product under the conditions shown below. Web draw an alkyl halide that would undergo an sn 2 reaction to. So, since tertiary carbocations are most stable of the three will undergo sn1 reaction easily. We can form terminal alkyne through e² elimination reaction. As the nucleophile comes in on one side of the substrate and bonds to the. Secondary alkyl halides react very slowly and tertiary alkyl halides react so, so. Web draw an alkyl halide that would undergo an sn2 reaction to yield this product under the conditions shown below. Web 1) structure of the alkyl halide. Structure of the alkyl halide (substrate) and s n 2 reaction rates. For the discussions on s n 2 mechanism so far, we focused on the reaction of methylbromide ch 3 br. Your solution’s ready to go! There are 2 steps to solve this one. Web your solution’s ready to go! Even though it is a primary alkyl halide, the rate of sn2 is way slower (about 10^5 times) compared to regular primary alkyl halides. Tertiary > secondary > primary. First of all we should… Web the sn2 mechanism proceeds through a concerted backside attack of a nucleophile upon an alkyl halide, and is fastest for methyl > primary > secondary> > 3° Sn1 reactions depend on the stability of the cation formed when the leaving group had left. Thf c h 3 c o 2 n a. Web the essential feature of the s n 2 mechanism is that it takes place in a single step, without intermediates, when the incoming nucleophile reacts with the alkyl halide or tosylate (the substrate) from a direction opposite the group that is displaced (the leaving group).Solved E Draw an alkyl halide that would undergo an SN2
Solved Draw an alkyl halide that would undergo an SN2
Solved Draw an alkyl halide that would undergo an SN2
Solved Draw an alkyl halide that would undergo an SN2
Solved Draw an alkyl halide that would undergo an SN2
Solved Draw an alkyl halide that would undergo an SN2
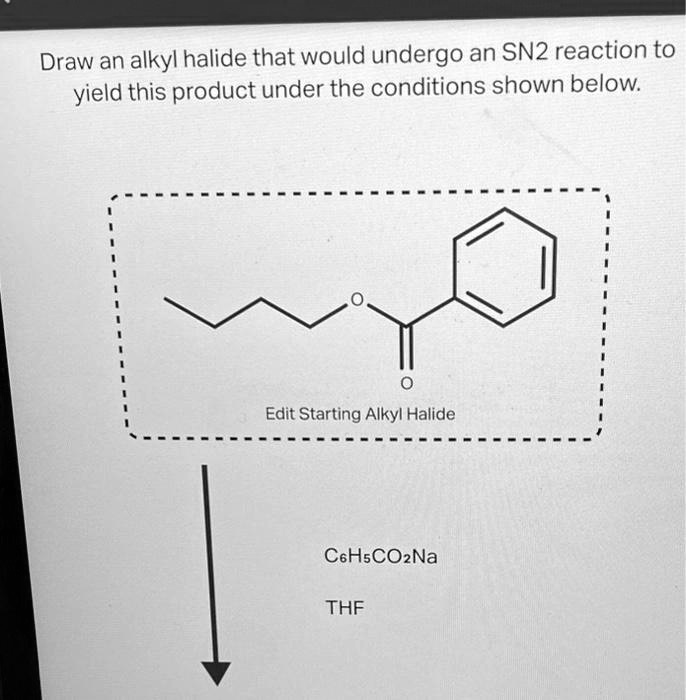
SOLVED Draw an alkyl halide that would undergo an SN2 reaction to
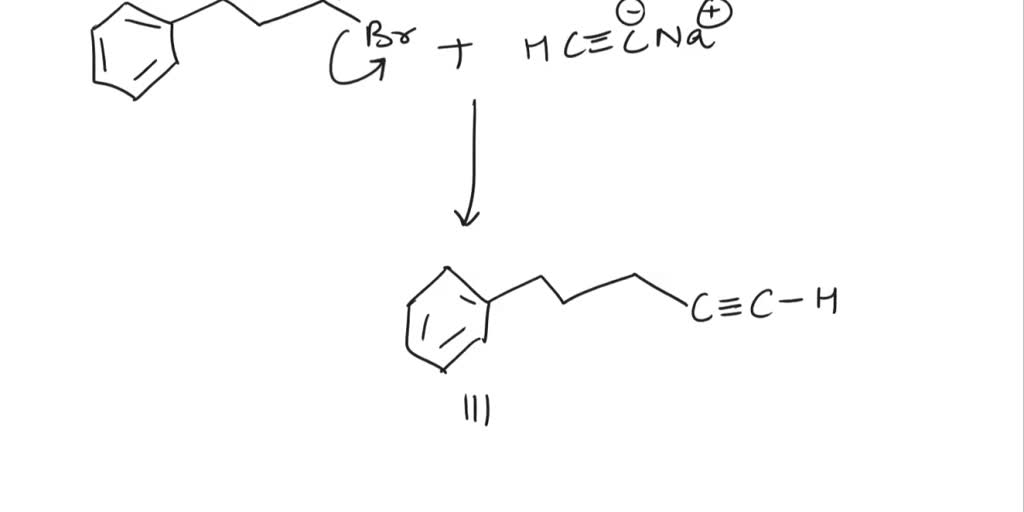
SOLVED Draw an alkyl halide that would undergo an SN2 reaction to
Solved Draw an alkyl halide that would undergo an SN2
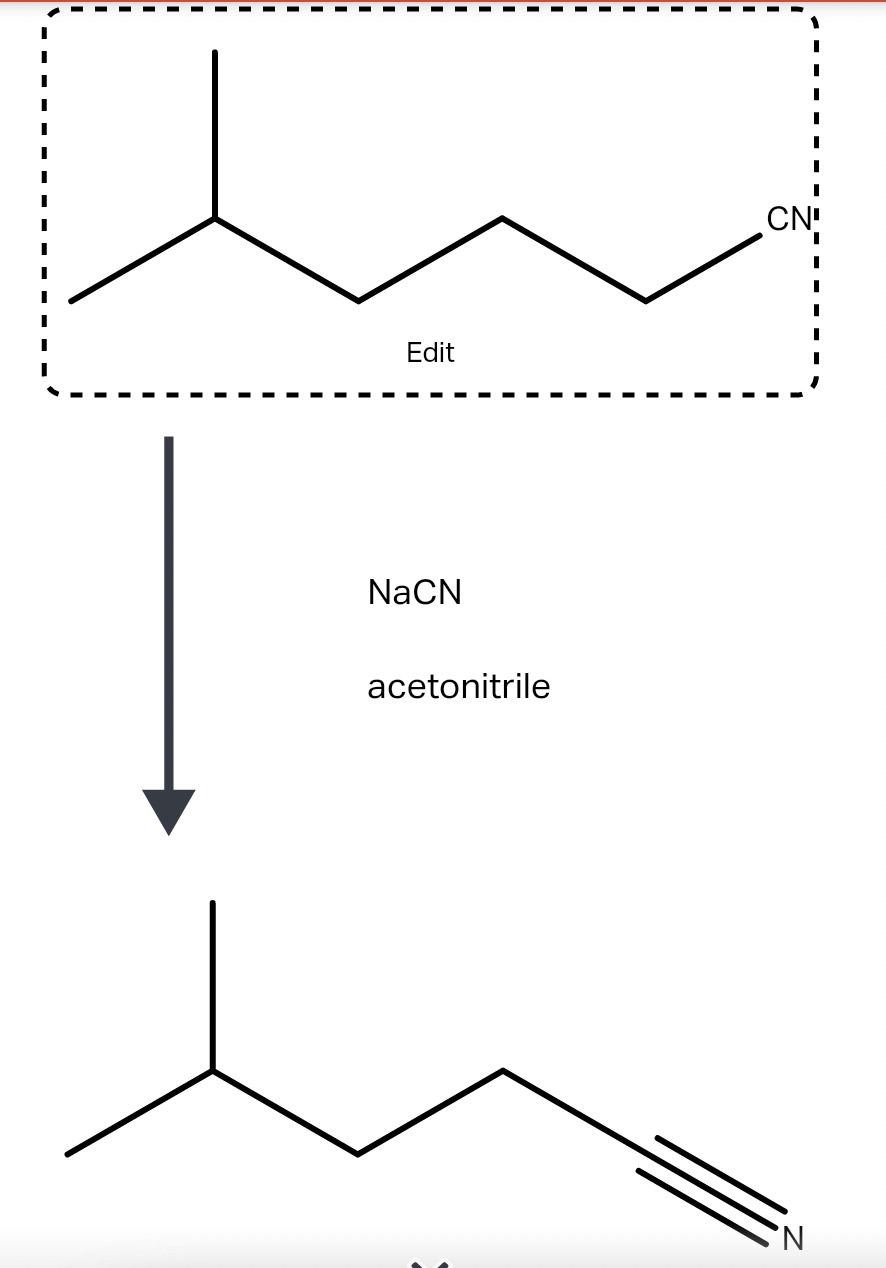
draw an alkyl halide that would undergo an sn2 reactio StudyX
We Can Form Terminal Alkyne Through E² Elimination Reaction.
Web Turns Out That The Methyl Halides And The Primary Alkyl Halide React The Fastest In An Sn2 Mechanism.
Yield This Product Under The Conditions Shown Below.
Web Your Solution’s Ready To Go!
Related Post: