Draw All Resonance Structures For The Acetate Ion Ch3Coo, Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule.
Draw All Resonance Structures For The Acetate Ion Ch3Coo - Web there is a third resonance form that can be drawn for the acetate ion hybrid. This means that it is of. Do not include overall ion. The structure shown below is structurally different from the ones shown above. Explicitly draw all h atoms. We start with a valid lewis structure and then follow these general rules. Web lewis dot of the acetate ion. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. For each resonance structure, assign formal charges to all atoms that have formal. For each resonance structure, assign formal charges to all atoms that have formal. The structure shown below is structurally different from the ones shown above. Include all valence lone pairs in your answer. Web there is a third resonance form that can be drawn for the acetate ion hybrid. For each resonance structure, assign formal charges to all atoms that have formal. Since all the atoms are in either period 1 or 2,. For each resonance structure, assign formal charges to all atoms that have formal. Web there is a third resonance form that can be drawn for the acetate ion hybrid. Do not include overall ion. We start with a valid lewis structure and then follow these general rules. Explicitly draw all h atoms. Include all valence lone pairs in your answer. For each resonance structure, assign formal charges to all atoms that have formal. 70 more lewis dot structures. Web there is a third resonance form that can be drawn for the acetate ion hybrid. This means that it is of. Do not include overall ion. Web lewis dot of the acetate ion. The structure shown below is structurally different from the ones shown above. Web there is a third resonance form that can be drawn for the acetate ion hybrid. We start with a valid lewis structure and then follow these general rules. Explicitly draw all h atoms. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. Web lewis dot of the acetate ion. 70 more lewis dot structures. Web there is a third resonance form that can be drawn for the acetate ion hybrid. 70 more lewis dot structures. For each resonance structure, assign formal charges to all atoms that have formal. For each resonance structure, assign formal charges to all atoms that have formal. Web there is a third resonance form that can be drawn for the acetate ion hybrid. Web lewis dot of the acetate ion. This means that it is of. For each resonance structure, assign formal charges to all atoms that have formal. Explicitly draw all h atoms. Include all valence lone pairs in your answer. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. For each resonance structure, assign formal charges to all atoms that have formal. The structure shown below is structurally different from the ones shown above. Include all valence lone pairs in your answer. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. This means that it is of. This means that it is of. We start with a valid lewis structure and then follow these general rules. Web there is a third resonance form that can be drawn for the acetate ion hybrid. For each resonance structure, assign formal charges to all atoms that have formal. Do not include overall ion. Web there is a third resonance form that can be drawn for the acetate ion hybrid. Explicitly draw all h atoms. For each resonance structure, assign formal charges to all atoms that have formal. Do not include overall ion. This means that it is of. For each resonance structure, assign formal charges to all atoms that have formal. This means that it is of. Web there is a third resonance form that can be drawn for the acetate ion hybrid. The structure shown below is structurally different from the ones shown above. Web lewis dot of the acetate ion. 70 more lewis dot structures. Include all valence lone pairs in your answer. Explicitly draw all h atoms. We start with a valid lewis structure and then follow these general rules.[Solved] Draw all resonance structures for the acetate ion

draw all resonance structures for the acetate ion ch3coo

Write resonance structures of CH3COO and show the movement of

Draw the Lewis structure including resonance structures for the acetate
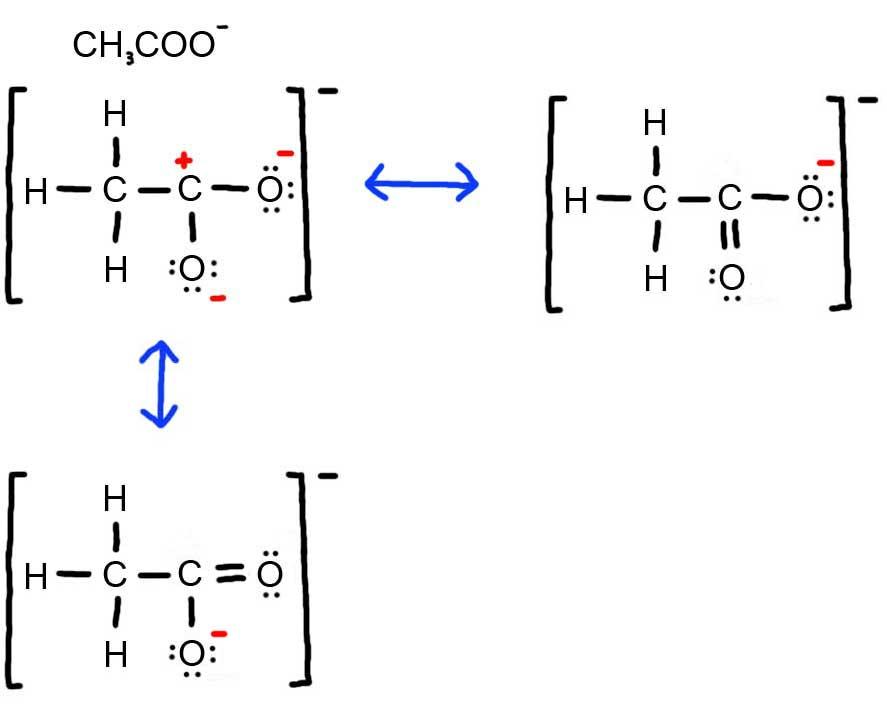
Resonance Chemistry LibreTexts
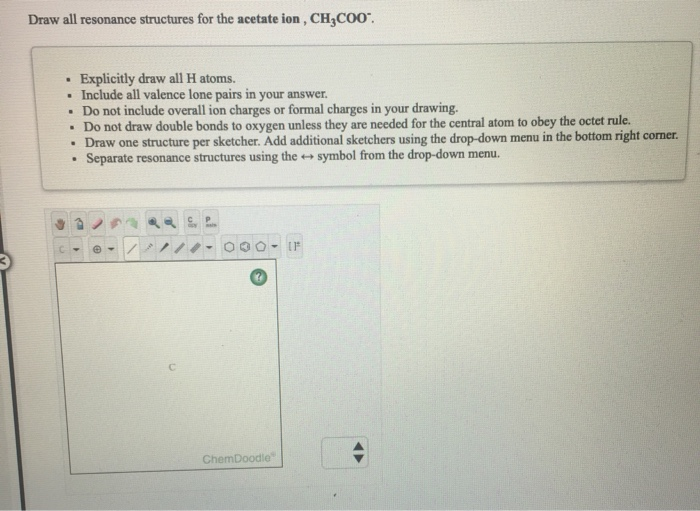
draw all resonance structures for the acetate ion ch3coo

SOLVED Draw the Lewis structure (including resonance structures) for
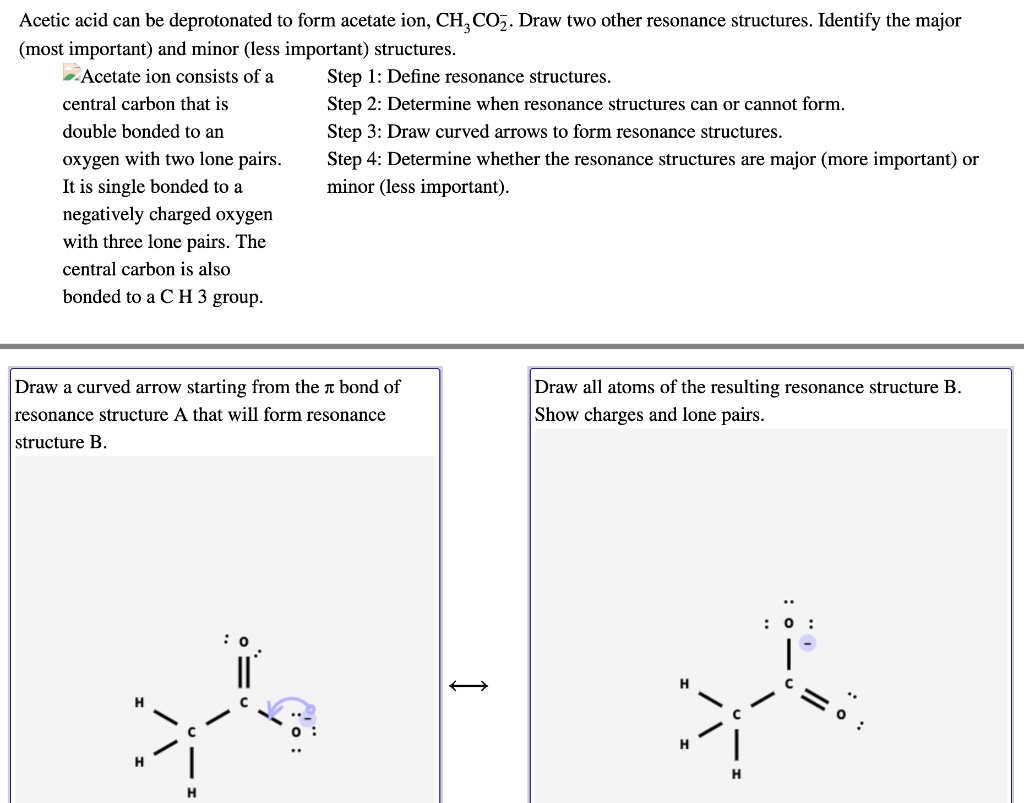
SOLVED Acetic acid can be deprotonated to form acetate ion, CH3CO2

draw all resonance structures for the acetate ion ch3coo
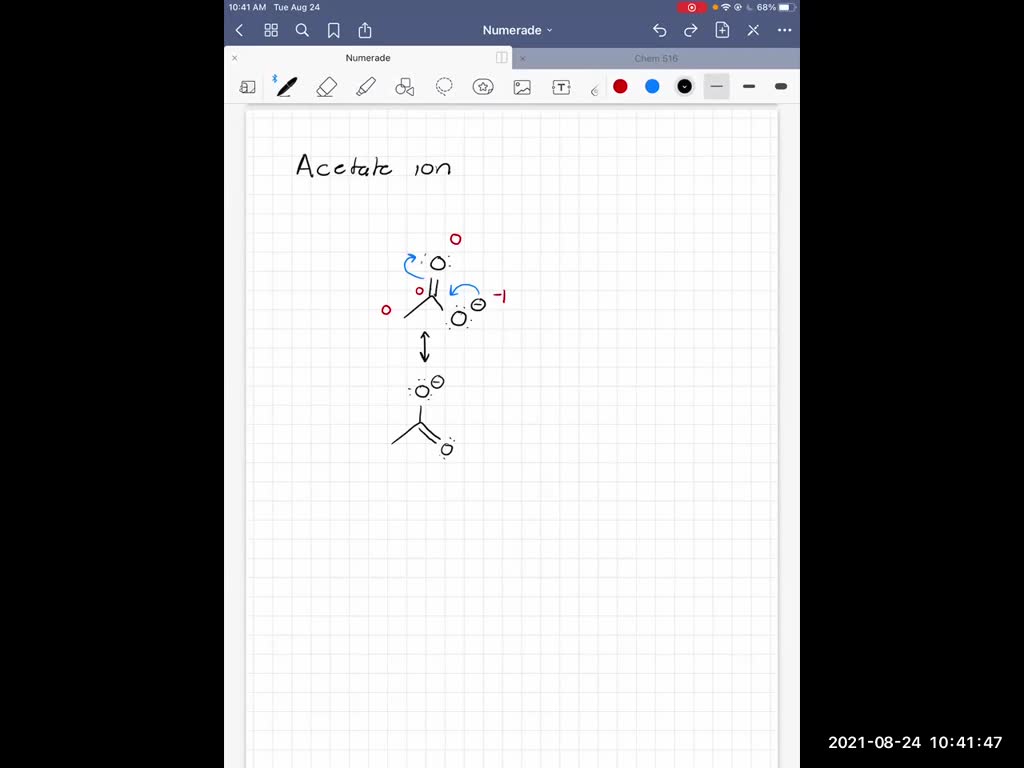
SOLVEDDraw the Lewis structure (including resonance structures) for
For Each Resonance Structure, Assign Formal Charges To All Atoms That Have Formal.
Since All The Atoms Are In Either Period 1 Or 2, This Molecule Will Adhere To The Octet Rule.
Do Not Include Overall Ion.
Related Post:
![[Solved] Draw all resonance structures for the acetate ion](https://media.cheggcdn.com/study/f65/f655d68e-d0a2-4d99-a9ae-76e4a3c7eea4/image)