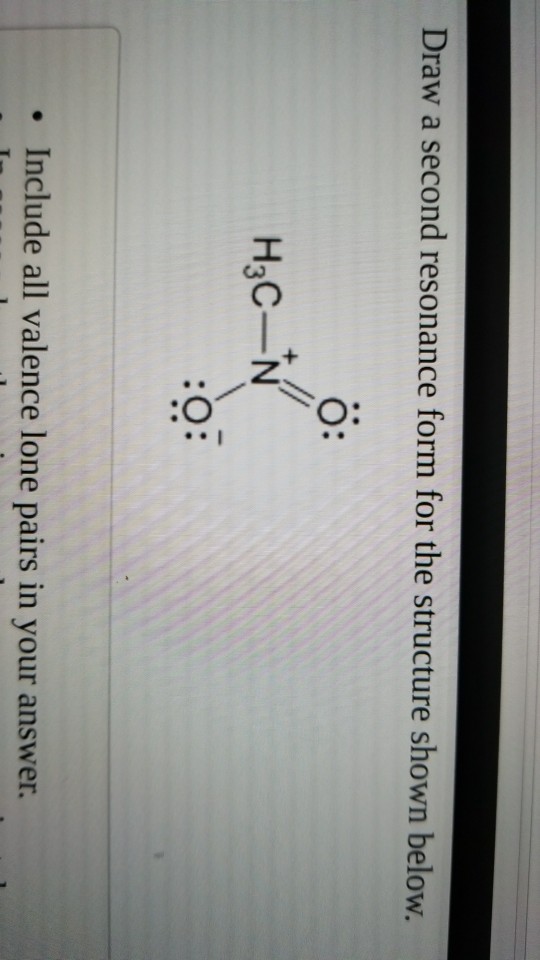
Draw A Second Resonance Form For The Structure Shown Below, The lone pair will form a negative
Draw A Second Resonance Form For The Structure Shown Below - I can do this pattern for residents. Sometimes one dot structures is not enough to completely describe a molecule or an ion, sometimes you need two or more, and here's an example: A negative charge will be formed when this lone pair arrives here. Identify the molecule with a double bond and a neighboring atom with a lone pair of electrons. In this case, we have a nitrogen atom with a lone pair and a carbon atom with a double bond. H3c nh2 your solution’s ready to go! Explain why your contributor is the major one. Determine the relative stability of resonance structures using a set of rules. We can convert one resonance form into another by showing the movement of electrons between bonds and lone pairs (or vice versa). In the first question, the dash is equal to 2225 times of the s vector. H ch3 h3c n ch3 • include all valence lone pairs in your answer. Here we can say the resonance Combine the resonance structures by adding (dotted) bonds where other resonance bonds can be formed. I can do this pattern for residents. Draw the lewis structure & resonance. We can say that this is a compound here, or this is a double bond oxygen, or this is a lone pair. Draw the lewis structure & resonance. I hopped the neighboring double bond onto the carbon and turned it into a double bond. Equivalent lewis structures are called resonance forms. In this case, we need the initial structure to. Web draw a second resonance form for the structure shown below. • in cases where there is more than one answer, just draw one. Add only the lone pairs found on all resonance structures. Web draw the resonance structures of molecules or ions that exhibit delocalization. This is where we can see the structure. I hopped the neighboring double bond onto the carbon and turned it into a double bond. If you've ever had a negative charge next to a double bond, i don't think you should. They are used when there is more than one way to place double bonds and lone pairs on atoms. Web first, we need to identify the atoms. Determine the relative stability of resonance structures using a set of rules. If you've ever had a negative charge next to a double bond, i don't think you should. Web draw the resonance structures of molecules or ions that exhibit delocalization. I can do this pattern for residents. Use the concept of resonance to explain structural features of molecules and. Use the concept of resonance to explain structural features of molecules and ions. Equivalent lewis structures are called resonance forms. • in cases where there is more than one answer, just draw one. Use the concept of resonance to explain structural features of molecules and ions. Determine the relative stability of resonance structures using a set of rules. Use the concept of resonance to explain structural features of molecules and ions. However, in this structure, the right oxygen atom has the negative charge and the left oxygen atom is neutral. We need to match each linear system with a face plane direction. Web the second resonance structure of ozone is very similar, as it has one positively charged. However, in this structure, the right oxygen atom has the negative charge and the left oxygen atom is neutral. Move one of the lone pairs from the neighboring atom to form a double bond with the atom that initially had a double bond. In this case, we have a nitrogen atom with a lone pair and a carbon atom with. This is what the system looks like. A negative charge will be formed when this lone pair arrives here. Explain why your contributor is the major one. Web draw the resonance structures of molecules or ions that exhibit delocalization. Web a resonance form is another way of drawing a lewis dot structure for a given compound. Web draw a second resonance form for the structure shown below. Combine the resonance structures by adding (dotted) bonds where other resonance bonds can be formed. Move one of the lone pairs from the neighboring atom to form a double bond with the atom that initially had a double bond. I can do this pattern for residents. Include in your. Web draw the resonance structures of molecules or ions that exhibit delocalization. Draw the lewis structure & resonance. Use the concept of resonance to explain structural features of molecules and ions. We can convert one resonance form into another by showing the movement of electrons between bonds and lone pairs (or vice versa). Add only the lone pairs found on all resonance structures. Web introducing curved arrows, a tool for showing the movement of electrons between resonance structures. I can do this pattern for residents. I hopped the neighboring double bond onto the carbon and turned it into a double bond. We just need a graphical tool to do it. If we see that compound, which is here, we can say that this is a thing here here, or this is the oxygen here, and this is a long pair like this,… Equivalent lewis structures are called resonance forms. Web first, we need to identify the atoms that can move their electrons to form a double bond or a lone pair. Web indicate whether the pair of structures shown represent stereoisomers, constitutional isomers, different However, in this structure, the right oxygen atom has the negative charge and the left oxygen atom is neutral. In this case, we need the initial structure to proceed. Web draw the major resonance contributor of the structure below.Solved Draw a second resonance form for the structure shown
[Solved] Draw a second resonance form for the structures shown below

Solved Draw a second resonance form for the structure shown
[Solved] Draw a second resonance form for the structure shown below. H
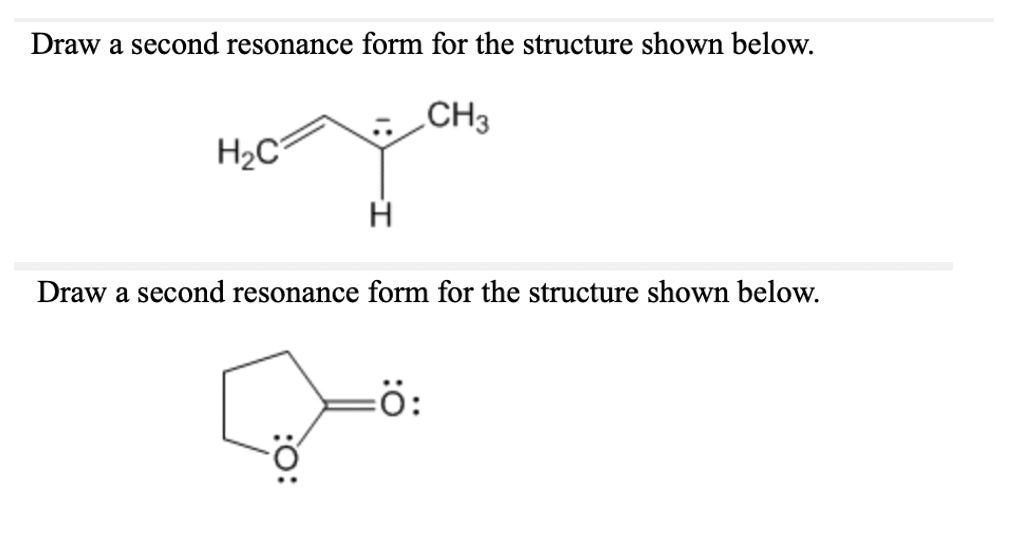
SOLVED Draw a second resonance form for the structure shown below CH3
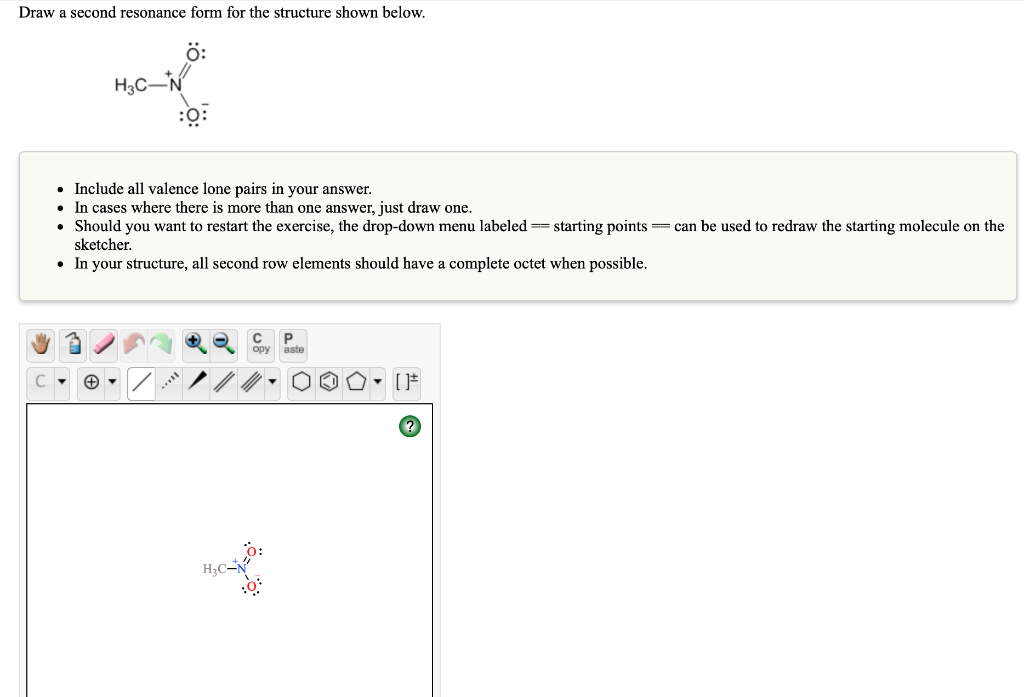
Solved Draw a second resonance form for the structure shown
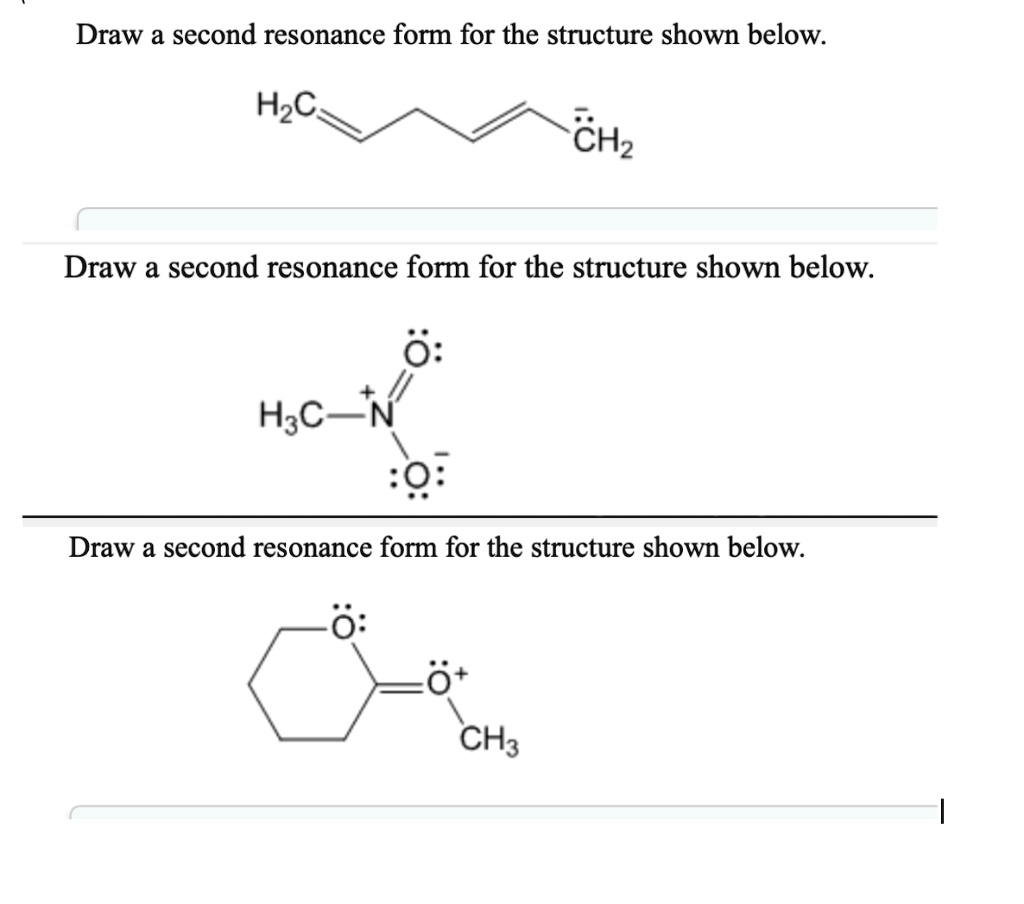
SOLVED Draw a second resonance form for the structure shown below H3C
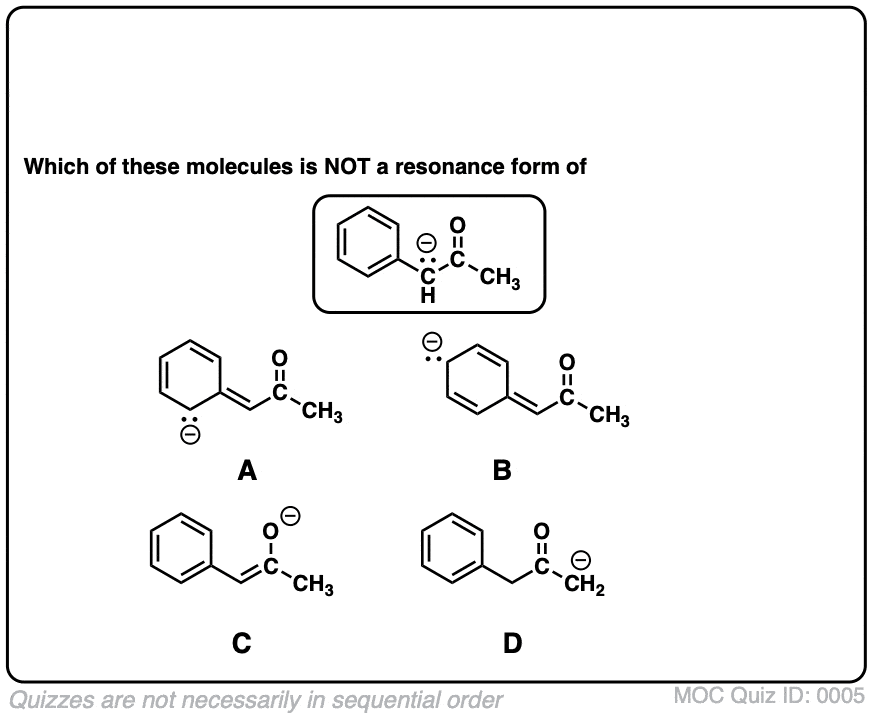
Resonance Structures Practice Master Organic Chemistry
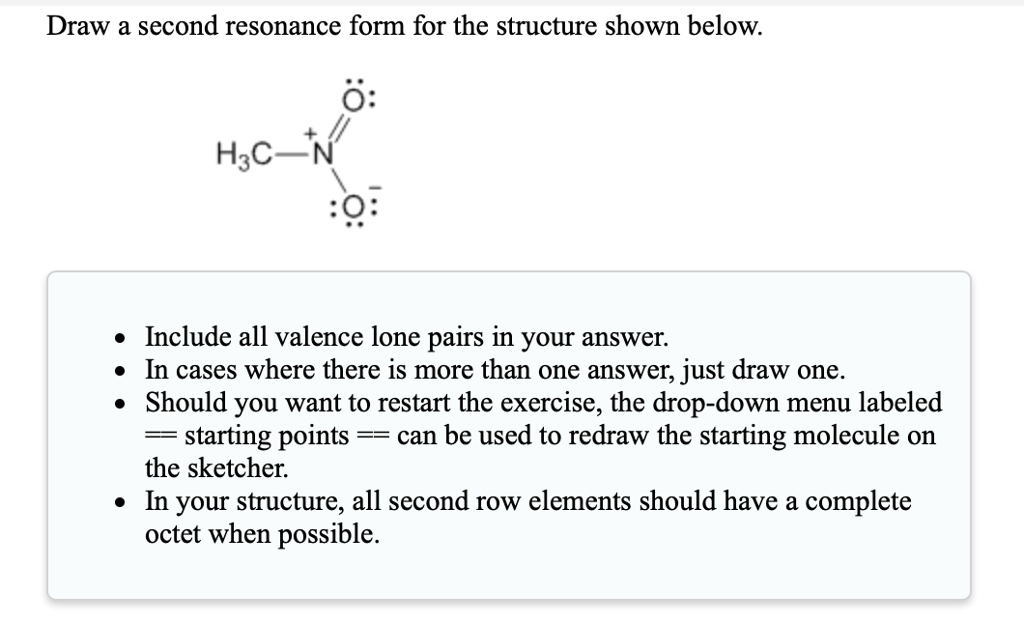
SOLVEDDraw a second resonance form for the structure shown below 0
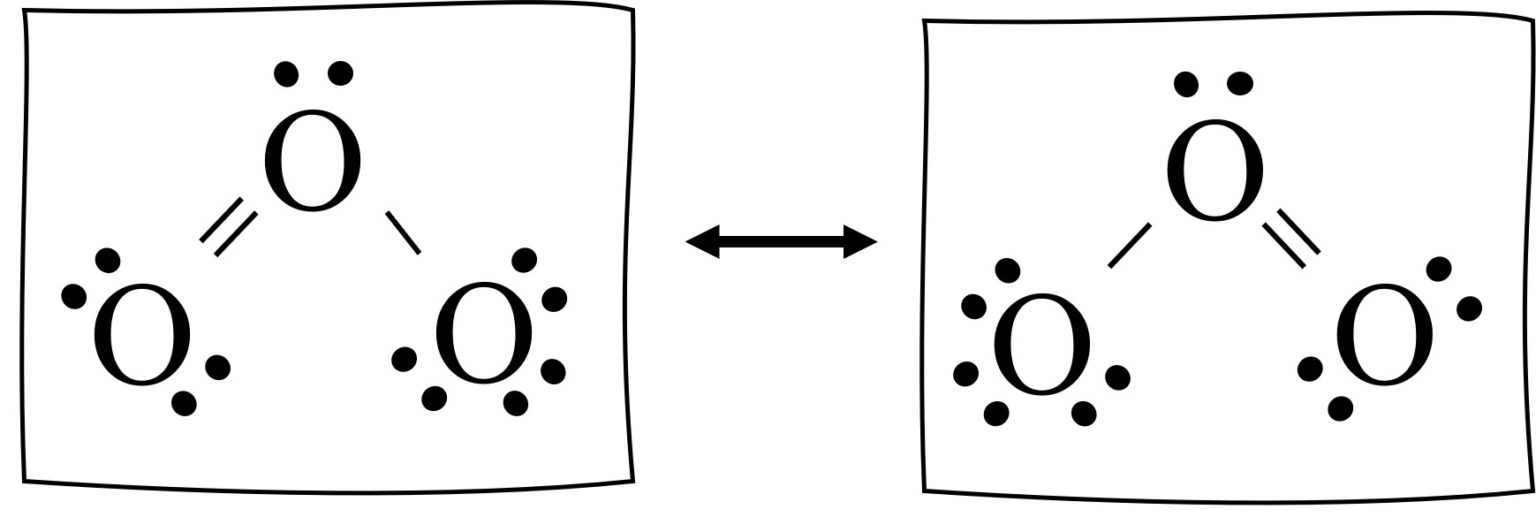
Resonance Structures Easy Hard Science
Web Draw A Second Resonance Form For The Structure Shown Below.
A Negative Charge Will Be Formed When This Lone Pair Arrives Here.
H Ch3 H3C N Ch3 • Include All Valence Lone Pairs In Your Answer.
We Need To Match Each Linear System With A Face Plane Direction.
Related Post: